Modulation of Asymmetric Division Diversity through Cytokinin and SPEECHLESS Regulatory Interactions in the Arabidopsis Stomatal Lineage
- PMID: 30197241
- PMCID: PMC6177308
- DOI: 10.1016/j.devcel.2018.08.007
Modulation of Asymmetric Division Diversity through Cytokinin and SPEECHLESS Regulatory Interactions in the Arabidopsis Stomatal Lineage
Abstract
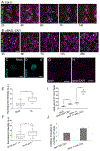
Coordinated growth of organs requires communication among cells within and between tissues. In plants, leaf growth is largely dictated by the epidermis; here, asymmetric and self-renewing divisions of the stomatal lineage create two essential cell types-pavement cells and guard cells-in proportions reflecting inputs from local, systemic, and environmental cues. The transcription factor SPEECHLESS (SPCH) is the prime regulator of divisions, but whether and how it is influenced by external cues to provide flexible development is enigmatic. Here, we show that the phytohormone cytokinin (CK) can act as an endogenous signal to affect the extent and types of stomatal lineage divisions and forms a regulatory circuit with SPCH. Local domains of low CK signaling are created by SPCH-dependent cell-type-specific activity of two repressive type-A ARABIDOPSIS RESPONSE REGULATORs (ARRs), ARR16 and ARR17, and two secreted peptides, CLE9 and CLE10, which, together with SPCH, can customize epidermal cell-type composition.
Keywords: Arabidopsis; SPEECHLESS; cytokinin signaling; growth regulation; meristemoid behavior; peptide signaling; stomatal development.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests:
The authors declare no competing interests.
Figures





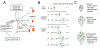
Similar articles
-
Direct roles of SPEECHLESS in the specification of stomatal self-renewing cells.Science. 2014 Sep 26;345(6204):1605-9. doi: 10.1126/science.1256888. Epub 2014 Sep 4. Science. 2014. PMID: 25190717 Free PMC article.
-
CDK8 of the mediator kinase module connects leaf development to the establishment of correct stomata patterning by regulating the levels of the transcription factor SPEECHLESS (SPCH).Plant Cell Environ. 2024 Dec;47(12):5237-5251. doi: 10.1111/pce.15102. Epub 2024 Aug 23. Plant Cell Environ. 2024. PMID: 39177450
-
Phosphorylation of Serine 186 of bHLH Transcription Factor SPEECHLESS Promotes Stomatal Development in Arabidopsis.Mol Plant. 2015 May;8(5):783-95. doi: 10.1016/j.molp.2014.12.014. Epub 2014 Dec 30. Mol Plant. 2015. PMID: 25680231
-
The plant stomatal lineage at a glance.J Cell Sci. 2019 Apr 26;132(8):jcs228551. doi: 10.1242/jcs.228551. J Cell Sci. 2019. PMID: 31028153 Free PMC article. Review.
-
Emerging roles of protein phosphorylation in regulation of stomatal development.J Plant Physiol. 2023 Jan;280:153882. doi: 10.1016/j.jplph.2022.153882. Epub 2022 Nov 26. J Plant Physiol. 2023. PMID: 36493667 Review.
Cited by
-
HSL1 and BAM1/2 impact epidermal cell development by sensing distinct signaling peptides.Nat Commun. 2022 Feb 15;13(1):876. doi: 10.1038/s41467-022-28558-4. Nat Commun. 2022. PMID: 35169143 Free PMC article.
-
Primary multistep phosphorelay activation comprises both cytokinin and abiotic stress responses: insights from comparative analysis of Brassica type-A response regulators.J Exp Bot. 2024 Oct 30;75(20):6346-6368. doi: 10.1093/jxb/erae335. J Exp Bot. 2024. PMID: 39171371 Free PMC article.
-
ABCG11 modulates cytokinin responses in Arabidopsis thaliana.Front Plant Sci. 2022 Jul 25;13:976267. doi: 10.3389/fpls.2022.976267. eCollection 2022. Front Plant Sci. 2022. PMID: 35958217 Free PMC article.
-
The TCP4 Transcription Factor Directly Activates TRICHOMELESS1 and 2 and Suppresses Trichome Initiation.Plant Physiol. 2019 Dec;181(4):1587-1599. doi: 10.1104/pp.19.00197. Epub 2019 Oct 1. Plant Physiol. 2019. PMID: 31575625 Free PMC article.
-
Multi-scale dynamics influence the division potential of stomatal lineage ground cells in Arabidopsis.Nat Commun. 2025 Mar 17;16(1):2612. doi: 10.1038/s41467-025-57730-9. Nat Commun. 2025. PMID: 40097420 Free PMC article.
References
-
- Bishopp A, Help H, El-Showk S, Weijers D, Scheres B, Friml J, Benková E, Mähönen AP, and Helariutta Y (2011a). A mutually inhibitory interaction between auxin and cytokinin specifies vascular pattern in roots. Curr. Biol 21, 917–926. - PubMed
-
- Bishopp A, Lehesranta S, Vaten A, Help H, El-Showk S, Scheres B, Helariutta K, Mahonen AP, Sakakibara H, and Helariutta Y (2011b). Phloem-transported cytokinin regulates polar auxin transport and maintains vascular pattern in the root meristem. Curr. Biol 21, 927–932. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

