Long-term metformin treatment in adolescents with obesity and insulin resistance, results of an open label extension study
- PMID: 30197416
- PMCID: PMC6129504
- DOI: 10.1038/s41387-018-0057-6
Long-term metformin treatment in adolescents with obesity and insulin resistance, results of an open label extension study
Abstract
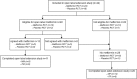
Background/objectives: Off-label metformin is nowadays frequently used for the treatment of obesity in adolescents. However, studies on long-term metformin treatment in adolescents with obesity are scarce. Therefore, an 18 month open label extension study following an 18 months randomized placebo-controlled trial (RCT) on the efficacy, safety, and tolerability of metformin in adolescents with obesity and insulin resistance was performed.
Subjects/methods: After completion of the RCT, metformin was offered to all participants with a body mass index standard deviation score (BMI-sds) > 2.3 and Homeostasis Model Assessment for Insulin Resistance (HOMA-IR) ≥ 3.4. Endpoints were change in BMI and HOMA-IR.
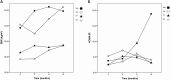
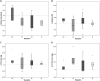
Results: Overall, 31/42 participants completed the extension study (74% girls, median age 14.8 (11.6 - 17.9), BMI 31.2 (22.3 - 45.1), HOMA-IR 3.4 (0.2 - 8.8)). At start, 22/42 (52.4%) participants were eligible for metformin of which 13 (59.0%) agreed with treatment. In participants who continued metformin, an increase was observed in BMI (+2.2 (+0.2 to +9.0)) and HOMA-IR (+13.7 (+1.6 to +48.3)). In metformin naive participants, BMI stabilized after an initial decrease (+0.5 (-2.1 to +5.1)). For HOMA-IR, a decrease was observed (-1.1 (-4.6 to +1.4)).
Conclusion: While metformin treatment in metformin naive participants seems to result in an initial decrease in BMI and HOMA-IR, there is no evidence for sustained effect after prolonged use in adolescents. Limited compliance and/or insufficient dose may explain the differences in long-term effects between adolescents and adults.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Long-term treatment with metformin in obese, insulin-resistant adolescents: results of a randomized double-blinded placebo-controlled trial.Nutr Diabetes. 2016 Aug 29;6(8):e228. doi: 10.1038/nutd.2016.37. Nutr Diabetes. 2016. PMID: 27571249 Free PMC article. Clinical Trial.
-
Metformin and placebo therapy both improve weight management and fasting insulin in obese insulin-resistant adolescents: a prospective, placebo-controlled, randomized study.Eur J Endocrinol. 2010 Oct;163(4):585-92. doi: 10.1530/EJE-10-0570. Epub 2010 Jul 16. Eur J Endocrinol. 2010. PMID: 20639355 Clinical Trial.
-
The effects of metformin on insulin resistance in overweight or obese children and adolescents: A PRISMA-compliant systematic review and meta-analysis of randomized controlled trials.Medicine (Baltimore). 2019 Jan;98(4):e14249. doi: 10.1097/MD.0000000000014249. Medicine (Baltimore). 2019. PMID: 30681616 Free PMC article.
-
The Effect of Eighteen-Month Metformin Treatment in Obese Adolescents: Comparison of Results Obtained in Daily Practice with Results from a Clinical Trial.J Obes. 2016;2016:7852648. doi: 10.1155/2016/7852648. Epub 2016 Dec 22. J Obes. 2016. PMID: 28101379 Free PMC article. Clinical Trial.
-
Efficacy and Safety of Metformin for Obesity: A Systematic Review.Pediatrics. 2021 Mar;147(3):e20201610. doi: 10.1542/peds.2020-1610. Pediatrics. 2021. PMID: 33608415
Cited by
-
Metformin Preserves β-Cell Compensation in Insulin Secretion and Mass Expansion in Prediabetic Nile Rats.Int J Mol Sci. 2021 Jan 3;22(1):421. doi: 10.3390/ijms22010421. Int J Mol Sci. 2021. PMID: 33401592 Free PMC article.
-
Anti-obesity pharmacotherapy for treatment of pediatric type 2 diabetes: Review of the literature and lessons learned from adults.Front Endocrinol (Lausanne). 2022 Oct 27;13:1043650. doi: 10.3389/fendo.2022.1043650. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36387846 Free PMC article. Review.
-
Diagnosis, treatment and prevention of type 2 diabetes mellitus in children and adolescents.World J Diabetes. 2021 Apr 15;12(4):344-365. doi: 10.4239/wjd.v12.i4.344. World J Diabetes. 2021. PMID: 33889284 Free PMC article. Review.
-
Metformin therapy in pediatric type 2 diabetes mellitus and its comorbidities: A review.Front Endocrinol (Lausanne). 2023 Feb 6;13:1072879. doi: 10.3389/fendo.2022.1072879. eCollection 2022. Front Endocrinol (Lausanne). 2023. PMID: 36814831 Free PMC article. Review.
-
Serum Concentrations and Dietary Intake of Vitamin B12 in Children and Adolescents on Metformin: A Case-Control Study.Int J Mol Sci. 2023 Feb 20;24(4):4205. doi: 10.3390/ijms24044205. Int J Mol Sci. 2023. PMID: 36835611 Free PMC article.
References
-
- World Health Organisation. Global and regional trends bij UN regions, 1990–2025 overweight: 1990–2015. http://apps.who.int/gho/data/view.main.NUTUNOVERWEIGHTv?lang=en. Accessed February 2017 (2016).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

