Sense and Insensibility - An Appraisal of the Effects of Clinical Anesthetics on Gastropod and Cephalopod Molluscs as a Step to Improved Welfare of Cephalopods
- PMID: 30197598
- PMCID: PMC6117391
- DOI: 10.3389/fphys.2018.01147
Sense and Insensibility - An Appraisal of the Effects of Clinical Anesthetics on Gastropod and Cephalopod Molluscs as a Step to Improved Welfare of Cephalopods
Abstract
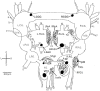
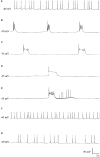
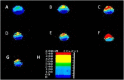
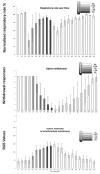
Recent progress in animal welfare legislation stresses the need to treat cephalopod molluscs, such as Octopus vulgaris, humanely, to have regard for their wellbeing and to reduce their pain and suffering resulting from experimental procedures. Thus, appropriate measures for their sedation and analgesia are being introduced. Clinical anesthetics are renowned for their ability to produce unconsciousness in vertebrate species, but their exact mechanisms of action still elude investigators. In vertebrates it can prove difficult to specify the differences of response of particular neuron types given the multiplicity of neurons in the CNS. However, gastropod molluscs such as Aplysia, Lymnaea, or Helix, with their large uniquely identifiable nerve cells, make studies on the cellular, subcellular, network and behavioral actions of anesthetics much more feasible, particularly as identified cells may also be studied in culture, isolated from the rest of the nervous system. To date, the sorts of study outlined above have never been performed on cephalopods in the same way as on gastropods. However, criteria previously applied to gastropods and vertebrates have proved successful in developing a method for humanely anesthetizing Octopus with clinical doses of isoflurane, i.e., changes in respiratory rate, color pattern and withdrawal responses. However, in the long term, further refinements will be needed, including recordings from the CNS of intact animals in the presence of a variety of different anesthetic agents and their adjuvants. Clues as to their likely responsiveness to other appropriate anesthetic agents and muscle relaxants can be gained from background studies on gastropods such as Lymnaea, given their evolutionary history.
Keywords: behavior; cephalopods; clinical anesthetics; gastropods; identified neurons.
Figures




References
-
- Ahmed I. A. (1995). Effects of General Anaesthetics and Other Pharmacological agents on Intracellular Calcium Levels in Identified Molluscan Neurons. Ph.D. thesis, University of Leeds, Leeds.
-
- Andrews P. L. R., Darmaillacq A. S., Dennison N., Gleadall I. G., Hawkins P., Messenger J. B., et al. (2013). The identification and management of pain, suffering and distress in cephalopods, including anaesthesia, analgesia and humane killing. J. Exp. Mar. Biol. Ecol. 447 46–64. 10.1016/j.jembe.2013.02.010 - DOI
-
- Andrews P. L. R., Tansey T. (1981). The effects of some anaesthetic agents in Octopus vulgaris. Comp. Biochem. Physiol. C Comp. Pharmacol. 70 241–247. 10.1016/0306-4492(81)90057-5 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

