Role of Short Chain Fatty Acids in Controlling Tregs and Immunopathology During Mucosal Infection
- PMID: 30197637
- PMCID: PMC6117408
- DOI: 10.3389/fmicb.2018.01995
Role of Short Chain Fatty Acids in Controlling Tregs and Immunopathology During Mucosal Infection
Abstract
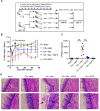
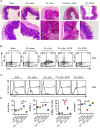
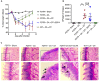
Interactions between mucosal tissues and commensal microbes control appropriate host immune responses and inflammation, but very little is known about these interactions. Here we show that the depletion of resident bacteria using antibiotics (Abx) causes oral and gut immunopathology during oropharyngeal candidiasis (OPC) infection. Antibiotic treatment causes reduction in the frequency of Foxp3+ regulatory cells (Tregs) and IL-17A producers, with a concomitant increase in oral tissue pathology. While C. albicans (CA) is usually controlled in the oral cavity, antibiotic treatment led to CA dependent oral and gut inflammation. A combination of short chain fatty acids (SCFA) controlled the pathology in Abx treated mice, correlating to an increase in the frequency of Foxp3+, IL-17A+, and Foxp3+IL-17A+ double positive (Treg17) cells in tongue and oral draining lymph nodes. However, SCFA treatment did not fully reverse the gut inflammation suggesting that resident microbiota have SCFA independent homeostatic mechanisms in gut mucosa. We also found that SCFA potently induce Foxp3 and IL-17A expression in CD4+ T cells, depending on the cytokine milieu in vitro. Depletion of Tregs alone in FDTR mice recapitulated oral inflammation in CA infected mice, showing that Abx mediated reduction of Tregs was involved in infection induced pathology. SCFA did not control inflammation in Treg depleted mice in CA infected FDTR mice, showing that Foxp3+ T cell induction was required for the protective effect mediated by SCFA. Taken together, our data reveal that SCFA derived from resident bacteria play a critical role in controlling immunopathology by regulating T cell cytokines during mucosal infections. This study has broader implications on protective effects of resident microbiota in regulating pathological infections.
Keywords: SCFA; Candida; Foxp3; Th17; Treg; oral-microbiome; oral-mucosa.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

