Hepatitis C Virus Envelope Glycoproteins: A Balancing Act of Order and Disorder
- PMID: 30197646
- PMCID: PMC6117417
- DOI: 10.3389/fimmu.2018.01917
Hepatitis C Virus Envelope Glycoproteins: A Balancing Act of Order and Disorder
Abstract
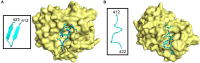
Chronic hepatitis C virus infection often leads to liver cirrhosis and primary liver cancer. In 2015, an estimated 71 million people were living with chronic HCV. Although infection rates have decreased in many parts of the world over the last several decades, incidence of HCV infection doubled between 2010 and 2014 in the United States mainly due to increases in intravenous drug use. The approval of direct acting antiviral treatments is a necessary component in the elimination of HCV, but inherent barriers to treatment (e.g., cost, lack of access to healthcare, adherence to treatment, resistance, etc.) prevent dramatic improvements in infection rates. An effective HCV vaccine would significantly slow the spread of the disease. Difficulties in the development of an HCV culture model system and expression of properly folded- and natively modified-HCV envelope glycoproteins E1 and E2 have hindered vaccine development efforts. The recent structural and biophysical studies of these proteins have demonstrated that the binding sites for the cellular receptor CD-81 and neutralizing antibodies are highly flexible in nature, which complicate vaccine design. Furthermore, the interactions between E1 and E2 throughout HCV infection is poorly understood, and structural flexibility may play a role in shielding antigenic epitopes during infection. Here we discuss the structural complexities of HCV E1 and E2.
Keywords: E1; E2; HCV; envelope glycoprotein; hepatitis C virus; vaccine design.
Figures



References
-
- National Academies of Sciences E, Medicine Eliminating the Public Health Problem of Hepatitis B and C in the United States: Phase One Report. Washington, DC: The National Academies Press; (2016). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

