Synergistic antitumor effect of suberoylanilide hydroxamic acid and cisplatin in osteosarcoma cells
- PMID: 30197679
- PMCID: PMC6126343
- DOI: 10.3892/ol.2018.9224
Synergistic antitumor effect of suberoylanilide hydroxamic acid and cisplatin in osteosarcoma cells
Abstract
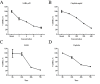
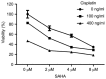
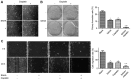
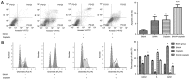
Cisplatin, as a first-line chemotherapy drug, has been widely applied for therapy of osteosarcoma. However, its application is limited by drug resistance and serious side effects, including nephrotoxicity and ototoxicity. Suberoylanilide hydroxamic acid (SAHA) is a newly developed histone deacetylase (HDAC) inhibitor, which is the first Food and Drug Administration-approved HDAC inhibitor for the treatment of cutaneous manifestations of T-cell lymphoma. However, SAHA as a monotherapy was revealed to be limited, particularly in solid tumors. In the present study, 143B osteosarcoma cells were treated with multiple concentrations of SAHA or cisplatin, either alone or combined. The morphological characteristics of the treated cells were observed using an inverted microscope. The cytotoxicity effects of the combination of SAHA and cisplatin on 143B cells were analyzed by MTT assay, colony formation assay, wound healing cell migration assay, cell apoptosis assay and cell cycle analysis. Western blot analysis was performed to detect the protein expression levels of B cell lymphoma-2 (Bcl-2)-associated X protein (Bax), Bcl-2, cleaved-caspase-3, cleaved-caspase-8 and cleaved-poly (ADP-ribose) polymerase (PARP). The experimental data indicated that the inhibition of cell proliferation in the combination group was significantly increased compared with that in single drug groups. Expression levels of pro-apoptotic protein were upregulated, whereas anti-apoptotic Bcl-2 was downregulated significantly in 143B cells following SAHA/cisplatin treatment. Taken together, the results revealed that the combination of SAHA and cisplatin inhibited the proliferation of 143B cells and induced their apoptosis synergistically, and this effectiveness may be mediated by caspase activation.
Keywords: cisplatin; drug combination; osteosarcoma; suberoylanilide hydroxamic acid; synergistic effect.
Figures
Similar articles
-
The HDAC Inhibitor, SAHA, Combined with Cisplatin Synergistically Induces Apoptosis in Alpha-fetoprotein-producing Hepatoid Adenocarcinoma Cells.Acta Histochem Cytochem. 2019 Feb 28;52(1):1-8. doi: 10.1267/ahc.18044. Epub 2019 Feb 23. Acta Histochem Cytochem. 2019. PMID: 30923410 Free PMC article.
-
Histone deacetylase inhibitor, suberoylanilide hydroxamic acid (SAHA), enhances anti-tumor effects of the poly (ADP-ribose) polymerase (PARP) inhibitor olaparib in triple-negative breast cancer cells.Breast Cancer Res. 2015 Mar 7;17:33. doi: 10.1186/s13058-015-0534-y. Breast Cancer Res. 2015. PMID: 25888415 Free PMC article.
-
Effects of suberoylanilide hydroxamic acid (SAHA) combined with paclitaxel (PTX) on paclitaxel-resistant ovarian cancer cells and insights into the underlying mechanisms.Cancer Cell Int. 2014 Nov 26;14(1):112. doi: 10.1186/s12935-014-0112-x. eCollection 2014. Cancer Cell Int. 2014. PMID: 25546354 Free PMC article.
-
Rosmarinic Acid, a Component of Rosemary Tea, Induced the Cell Cycle Arrest and Apoptosis through Modulation of HDAC2 Expression in Prostate Cancer Cell Lines.Nutrients. 2018 Nov 16;10(11):1784. doi: 10.3390/nu10111784. Nutrients. 2018. PMID: 30453545 Free PMC article.
-
The effect of combined treatment with cisplatin and histone deacetylase inhibitors on HeLa cells.J Gynecol Oncol. 2010 Dec 30;21(4):262-8. doi: 10.3802/jgo.2010.21.4.262. Epub 2010 Dec 31. J Gynecol Oncol. 2010. PMID: 21278889 Free PMC article.
Cited by
-
Selective Targeting of Class I Histone Deacetylases in a Model of Human Osteosarcoma.Cancers (Basel). 2021 Aug 20;13(16):4199. doi: 10.3390/cancers13164199. Cancers (Basel). 2021. PMID: 34439353 Free PMC article.
-
The HDAC Inhibitor, SAHA, Combined with Cisplatin Synergistically Induces Apoptosis in Alpha-fetoprotein-producing Hepatoid Adenocarcinoma Cells.Acta Histochem Cytochem. 2019 Feb 28;52(1):1-8. doi: 10.1267/ahc.18044. Epub 2019 Feb 23. Acta Histochem Cytochem. 2019. PMID: 30923410 Free PMC article.
-
Sodium Butyrate Enhances the Cytotoxic Effect of Etoposide in HDACi-Sensitive and HDACi-Resistant Transformed Cells.Int J Mol Sci. 2023 Nov 2;24(21):15913. doi: 10.3390/ijms242115913. Int J Mol Sci. 2023. PMID: 37958894 Free PMC article.
-
The Histone Deacetylase Inhibitor Entinostat/Syndax 275 in Osteosarcoma.Adv Exp Med Biol. 2020;1257:75-83. doi: 10.1007/978-3-030-43032-0_7. Adv Exp Med Biol. 2020. PMID: 32483732 Review.
References
-
- Ferrari S, Smeland S, Mercuri M, Bertoni F, Longhi A, Ruggieri P, Alvegard TA, Picci P, Capanna R, Bernini G, et al. Neoadjuvant chemotherapy with high-dose Ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: A joint study by the Italian and Scandinavian Sarcoma Groups. J Clin Oncol. 2005;23:8845–8852. doi: 10.1200/JCO.2004.00.5785. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
