Integrin α2β1 decelerates proliferation, but promotes survival and invasion of prostate cancer cells
- PMID: 30197754
- PMCID: PMC6126696
- DOI: 10.18632/oncotarget.25945
Integrin α2β1 decelerates proliferation, but promotes survival and invasion of prostate cancer cells
Abstract
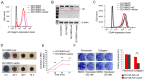
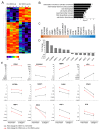
High expression level of integrin α2β1 is a hallmark of prostate cancer stem cell like cells. The role of this collagen receptor is controversial since it is down regulated in poorly differentiated carcinomas, but concomitantly proposed to promote metastasis. Here, we show that docetaxel resistant DU145 prostate cancer cells express high levels of α2β1 and that α2β1High subpopulation of DU145 cells proliferates slower than the cells representing α2β1Low subpopulation. To further study this initial observation we used Crispr/Cas9 technology to create an α2β1 negative DU145 cell line. Furthermore, we performed rescue experiment by transfecting α2 knockout cells with vector carrying α2 cDNA or with an empty vector for appropriate control. When these two cell lines were compared, α2β1 positive cells proliferated slower, were more resistant to docetaxel and also migrated more effectively on collagen and invaded faster through matrigel or collagen. Integrin α2β1 was demonstrated to be a positive regulator of p38 MAPK phosphorylation and a selective p38 inhibitor (SB203580) promoted proliferation and inhibited invasion. Effects of α2β1 integrin on the global gene expression pattern of DU145 cells in spheroid cultures were studied by RNA sequencing. Integrin α2β1 was shown to regulate several cancer progression related genes, most notably matrix metalloproteinase-1 (MMP-1), a recognized invasion promoting protein. To conclude, the fact that α2β1 decelerates cell proliferation may explain the dominance of α2β1 negative/low cells in primary sites of poorly differentiated carcinomas, while the critical role of α2β1 integrin in invasion stresses the importance of this adhesion receptor in cancer dissemination.
Keywords: cancer stem cells; cell adhesion; integrin; p38; prostate cancer.
Conflict of interest statement
CONFLICTS OF INTEREST The authors disclose no potential conflicts of interest.
Figures



References
-
- Heino J. Cellular signaling by collagen-binding integrins. Adv Exp Med Biol. 2014;819:143–55. - PubMed
-
- Sriramarao P, Mendler M, Bourdon MA. Endothelial cell attachment and spreading on human tenascin is mediated by alpha 2 beta 1 and alpha v beta 3 integrins. J Cell Sci. 1993;105:1001–12. - PubMed
-
- Guidetti G, Bertoni A, Viola M, Tira E, Balduini C, Torti M. The small proteoglycan decorin supports adhesion and activation of human platelets. Blood. 2002;100:1707–14. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

