Inositol 1,4,5-trisphosphate Receptor Mutations associated with Human Disease
- PMID: 30197841
- PMCID: PMC6128530
Inositol 1,4,5-trisphosphate Receptor Mutations associated with Human Disease
Abstract
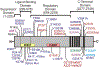
Calcium release into the cytosol via the inositol 1,4,5-trisphosphate receptor (IP3R) calcium channel is important for a variety of cellular processes. As a result, impairment or inhibition of this release can result in disease. Recently, mutations in all four domains of the IP3R have been suggested to cause diseases such as ataxia, cancer, and anhidrosis; however, most of these mutations have not been functionally characterized. In this review we summarize the reported mutations, as well as the associated symptoms. Additionally, we use clues from transgenic animals, receptor stoichiometry, and domain location of mutations to speculate on the effects of individual mutations on receptor structure and function and the overall mechanism of disease.
Figures

References
-
- Ando H, Mizutani A, Kiefer H, Tsuzurugi D, Michikawa T and Mikoshiba K (2006) ‘IRBIT suppresses IP3 receptor activity by competing with IP3 for the common binding site on the IP3 receptor’, Mol Cell, 22(6), pp. 795–806. - PubMed
-
- Ando H, Mizutani A, Matsu-ura T and Mikoshiba K (2003) ‘IRBIT, a novel inositol 1,4,5-trisphosphate (IP3) receptor-binding protein, is released from the IP3 receptor upon IP3 binding to the receptor’, J Biol Chem, 278(12), pp. 10602–12. - PubMed
-
- Barresi S, Niceta M, Alfieri P, Brankovic V, Piccini G, Bruselles A, Barone MR, Cusmai R, Tartaglia M, Bertini E and Zanni G (2017) ‘Mutations in the IRBIT domain of ITPR1 are a frequent cause of autosomal dominant nonprogressive congenital ataxia’, Clin Genet, 91(1), pp. 86–91. - PubMed
-
- Berridge MJ (1993) ‘Inositol trisphosphate and calcium signalling’, Nature, 361(6410), pp. 315–25. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
