Apolipoprotein CII Amyloidosis Associated With p.Lys41Thr Mutation
- PMID: 30197986
- PMCID: PMC6127408
- DOI: 10.1016/j.ekir.2018.04.009
Apolipoprotein CII Amyloidosis Associated With p.Lys41Thr Mutation
Abstract
Introduction: Apolipoprotein CII amyloidosis (AApoCII) is a rare form of amyloidosis. Here, we report a novel mutation associated with AApoCII amyloidosis in 5 patients and describe their clinical, renal biopsy, and mass spectrometry findings.
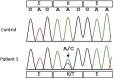
Methods: Five patients with renal AApoCII p.Lys41Thr amyloidosis were identified from our amyloid mass spectrometry cohort. Clinical features, kidney biopsy, and mass spectrometry findings were analyzed in this rare type of amyloidosis.
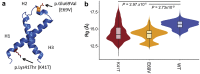
Results: The patients were older adults (mean age of 71.6 years at diagnosis), presented with nephrotic-range proteinuria, and often had declining renal function. All renal biopsy specimens showed massive mesangial nodules composed of weakly eosinophilic, periodic acid-Schiff negative, Congo red-positive amyloid deposits. There were no interstitial, vascular, or medullary deposits. In all cases, immunofluorescence studies were negative for Igs and electron microscopy showed amyloid fibrils. Proteomic analysis of Congo red-positive amyloid deposits detected large amounts of apolipoprotein CII (APOC2) protein. We also detected APOC2 p.Lys41Thr mutant protein in amyloid deposits of all patients. DNA sequencing in 1 patient confirmed the presence of the mutation. Both mutant and wild-type forms of APOC2 were detected in amyloid deposits of all patients. Molecular dynamic simulations showed the variant mediating a collapse of the native structure of APOC2, thereby destabilizing the protein.
Conclusion: We propose that AApoCII p.Lys41Thr amyloidosis is a new form of amyloidosis seen in elderly individuals, histologically exhibiting massive glomerular involvement, leading to nephrotic-range proteinuria and progressive chronic kidney disease.
Keywords: amyloidosis; apolipoprotein CII; kidney; mutation; p.Lys41Thr; proteomics.
Figures


References
-
- Dember L.M. Amyloidosis-associated kidney disease. J Am Soc Nephrol. 2006;17:3458–3471. - PubMed
-
- Merlini G., Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003;349:583–596. - PubMed
-
- Picken M.M. New insights into systemic amyloidosis: the importance of diagnosis of specific type. Curr Opin Nephrol Hypertens. 2007;16:196–203. - PubMed
-
- Vigushin D.M., Gough J., Allan D. Familial nephropathic systemic amyloidosis caused by apolipoprotein Al variant Arg26. Q J Med. 1994;87:149–154. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

