Development and Implementation of a Subcutaneous Insulin Clinical Decision Support Tool for Hospitalized Patients
- PMID: 30198324
- PMCID: PMC6501530
- DOI: 10.1177/1932296818798036
Development and Implementation of a Subcutaneous Insulin Clinical Decision Support Tool for Hospitalized Patients
Abstract
Background: Insulin is one of the highest risk medications used in hospitalized patients. Multiple complex factors must be considered in determining a safe and effective insulin regimen. We sought to develop a computerized clinical decision support (CDS) tool to assist hospital-based clinicians in insulin management.
Methods: Adapting existing clinical practice guidelines for inpatient glucose management, a design team selected, configured, and implemented a CDS tool to guide subcutaneous insulin dosing in non-critically ill hospitalized patients at two academic medical centers that use the EpicCare® electronic medical record (EMR). The Agency for Healthcare Research and Quality (AHRQ) best practices in CDS design and implementation were followed.
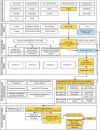
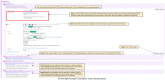
Results: A CDS tool was developed in the form of an EpicCare SmartForm, which generates an insulin regimen by integrating information about the patient's body weight, diabetes type, home and hospital insulin requirements, and nutritional status. Total daily recommended insulin doses are distributed into respective basal and nutritional doses with a tailored correctional insulin scale. Preimplementation, several approaches were used to communicate this new tool to clinicians, including emails, lectures, and videos. Postimplementation, a support team was available to address user technical issues. Feedback from stakeholders has been used to continuously refine the tool. Inclusion of the programming in the EMR vendor's community library has allowed dissemination of the tool outside our institution.
Conclusions: We have developed an EMR-based tool to guide SQ insulin dosing in non-critically ill hospitalized patients. Further studies are needed to evaluate adoption and clinical effectiveness of this intervention.
Keywords: clinical decision support systems; diabetes mellitus; hospital management insulin; subcutaneous (SQ).
Conflict of interest statement
Figures



Similar articles
-
Impact of errors in paper-based and computerized diabetes management with decision support for hospitalized patients with type 2 diabetes. A post-hoc analysis of a before and after study.Int J Med Inform. 2016 Jun;90:58-67. doi: 10.1016/j.ijmedinf.2016.03.007. Epub 2016 Mar 23. Int J Med Inform. 2016. PMID: 27103198
-
Approach to the adult hospitalized patient on an insulin pump.J Hosp Med. 2013 Dec;8(12):721-7. doi: 10.1002/jhm.2109. Epub 2013 Nov 13. J Hosp Med. 2013. PMID: 24227761 Review.
-
Design and implementation of a clinical decision support tool for primary palliative Care for Emergency Medicine (PRIM-ER).BMC Med Inform Decis Mak. 2020 Jan 28;20(1):13. doi: 10.1186/s12911-020-1021-7. BMC Med Inform Decis Mak. 2020. PMID: 31992301 Free PMC article. Clinical Trial.
-
A survey on the applications of implantable micropump systems in drug delivery.Curr Drug Deliv. 2014;11(1):123-31. doi: 10.2174/156720181101140212165729. Curr Drug Deliv. 2014. PMID: 24533725 Review.
-
Decision support systems in clinical practice: The case of venous thromboembolism prevention.Int J Risk Saf Med. 2015;27 Suppl 1:S104-5. doi: 10.3233/JRS-150709. Int J Risk Saf Med. 2015. PMID: 26639683
Cited by
-
Clinical decision support to improve management of diabetes and dysglycemia in the hospital: a path to optimizing practice and outcomes.BMJ Open Diabetes Res Care. 2021 Jan;9(1):e001557. doi: 10.1136/bmjdrc-2020-001557. BMJ Open Diabetes Res Care. 2021. PMID: 33462075 Free PMC article.
-
Efficacy and safety of basal-bolus insulin at 1:1.5 ratio compared to 1:1 ratio using a weight-based initiation and titration (WIT2) algorithm in hospitalized patients with type 2 Diabetes: a multicenter, randomized, clinical study.Diabetol Metab Syndr. 2023 Nov 27;15(1):243. doi: 10.1186/s13098-023-01193-9. Diabetol Metab Syndr. 2023. PMID: 38008775 Free PMC article.
-
Clinical Decision Support for Diabetes Care in the Hospital: A Time for Change Toward Improvement of Management and Outcomes.J Diabetes Sci Technol. 2022 May;16(3):771-774. doi: 10.1177/1932296820982661. Epub 2021 Jan 7. J Diabetes Sci Technol. 2022. PMID: 33412952 Free PMC article.
-
A Scoping Review of Integrated Medical Devices and Clinical Decision Support in the Acute Care Setting.Appl Clin Inform. 2022 Oct;13(5):1223-1236. doi: 10.1055/s-0042-1759513. Epub 2022 Dec 28. Appl Clin Inform. 2022. PMID: 36577503 Free PMC article.
-
Association Between Daily Insulin Dose Adjustments and Glycemic Control in Noncritically Ill Hospitalized Hyperglycemic Patients: A Retrospective Cohort Study.Endocr Pract. 2025 May;31(5):557-563. doi: 10.1016/j.eprac.2025.01.008. Epub 2025 Jan 28. Endocr Pract. 2025. PMID: 39884508
References
-
- Brutsaert E, Carey M, Zonszein J. The clinical impact of inpatient hypoglycemia. J Diabetes Complications. 2014;28(4):565-572. - PubMed
-
- Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978-982. - PubMed
-
- Martin WG, Galligan J, Simpson S, Greenaway T, Burgess J. Admission blood glucose predicts mortality and length of stay in patients admitted through the emergency department. Intern Med J. 2015;45(9):916-924. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

