Pseudomonas aeruginosa utilizes host polyunsaturated phosphatidylethanolamines to trigger theft-ferroptosis in bronchial epithelium
- PMID: 30198910
- PMCID: PMC6159971
- DOI: 10.1172/JCI99490
Pseudomonas aeruginosa utilizes host polyunsaturated phosphatidylethanolamines to trigger theft-ferroptosis in bronchial epithelium
Abstract
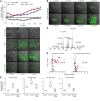
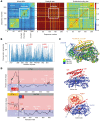
Ferroptosis is a death program executed via selective oxidation of arachidonic acid-phosphatidylethanolamines (AA-PE) by 15-lipoxygenases. In mammalian cells and tissues, ferroptosis has been pathogenically associated with brain, kidney, and liver injury/diseases. We discovered that a prokaryotic bacterium, Pseudomonas aeruginosa, that does not contain AA-PE can express lipoxygenase (pLoxA), oxidize host AA-PE to 15-hydroperoxy-AA-PE (15-HOO-AA-PE), and trigger ferroptosis in human bronchial epithelial cells. Induction of ferroptosis by clinical P. aeruginosa isolates from patients with persistent lower respiratory tract infections was dependent on the level and enzymatic activity of pLoxA. Redox phospholipidomics revealed elevated levels of oxidized AA-PE in airway tissues from patients with cystic fibrosis (CF) but not with emphysema or CF without P. aeruginosa. We believe that the evolutionarily conserved mechanism of pLoxA-driven ferroptosis may represent a potential therapeutic target against P. aeruginosa-associated diseases such as CF and persistent lower respiratory tract infections.
Keywords: Bacterial infections; Cell Biology; Infectious disease.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
- T32 AI060525/AI/NIAID NIH HHS/United States
- U19 AI068021/AI/NIAID NIH HHS/United States
- R01 HL097376/HL/NHLBI NIH HHS/United States
- R01 HL123771/HL/NHLBI NIH HHS/United States
- P30 DK072506/DK/NIDDK NIH HHS/United States
- R56 HL123771/HL/NHLBI NIH HHS/United States
- T32 GM007270/GM/NIGMS NIH HHS/United States
- R01 HL136143/HL/NHLBI NIH HHS/United States
- R01 NS061817/NS/NINDS NIH HHS/United States
- R01 HL142084/HL/NHLBI NIH HHS/United States
- P01 HL114453/HL/NHLBI NIH HHS/United States
- R01 HL081784/HL/NHLBI NIH HHS/United States
- UH2 HL123502/HL/NHLBI NIH HHS/United States
- R01 NS076511/NS/NINDS NIH HHS/United States
- R01 HL096376/HL/NHLBI NIH HHS/United States
- T32 HL129949/HL/NHLBI NIH HHS/United States
- R01 HL086884/HL/NHLBI NIH HHS/United States
- R01 HL098174/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

