Antigen-specific active immunotherapy for ovarian cancer
- PMID: 30199097
- PMCID: PMC6513204
- DOI: 10.1002/14651858.CD007287.pub4
Antigen-specific active immunotherapy for ovarian cancer
Abstract
Background: This is the second update of the review first published in the Cochrane Library (2010, Issue 2) and later updated (2014, Issue 9).Despite advances in chemotherapy, the prognosis of ovarian cancer remains poor. Antigen-specific active immunotherapy aims to induce tumour antigen-specific anti-tumour immune responses as an alternative treatment for ovarian cancer.
Objectives: Primary objective• To assess the clinical efficacy of antigen-specific active immunotherapy for the treatment of ovarian cancer as evaluated by tumour response measured by Response Evaluation Criteria In Solid Tumors (RECIST) and/or cancer antigen (CA)-125 levels, response to post-immunotherapy treatment, and survival differences◦ In addition, we recorded the numbers of observed antigen-specific humoral and cellular responsesSecondary objective• To establish which combinations of immunotherapeutic strategies with tumour antigens provide the best immunological and clinical results SEARCH METHODS: For the previous version of this review, we performed a systematic search of the Cochrane Central Register of Controlled Trials (CENTRAL; 2009, Issue 3), in the Cochrane Library, the Cochrane Gynaecological Cancer Group Specialised Register, MEDLINE and Embase databases, and clinicaltrials.gov (1966 to July 2009). We also conducted handsearches of the proceedings of relevant annual meetings (1996 to July 2009).For the first update of this review, we extended the searches to October 2013, and for this update, we extended the searches to July 2017.
Selection criteria: We searched for randomised controlled trials (RCTs), as well as non-randomised studies (NRSs), that included participants with epithelial ovarian cancer, irrespective of disease stage, who were treated with antigen-specific active immunotherapy, irrespective of type of vaccine, antigen used, adjuvant used, route of vaccination, treatment schedule, and reported clinical or immunological outcomes.
Data collection and analysis: Two reviews authors independently extracted the data. We evaluated the risk of bias for RCTs according to standard methodological procedures expected by Cochrane, and for NRSs by using a selection of quality domains deemed best applicable to the NRS.
Main results: We included 67 studies (representing 3632 women with epithelial ovarian cancer). The most striking observations of this review address the lack of uniformity in conduct and reporting of early-phase immunotherapy studies. Response definitions show substantial variation between trials, which makes comparison of trial results unreliable. Information on adverse events is frequently limited. Furthermore, reports of both RCTs and NRSs frequently lack the relevant information necessary for risk of bias assessment. Therefore, we cannot rule out serious biases in most of the included trials. However, selection, attrition, and selective reporting biases are likely to have affected the studies included in this review. GRADE ratings were high only for survival; for other primary outcomes, GRADE ratings were very low.The largest body of evidence is currently available for CA-125-targeted antibody therapy (17 studies, 2347 participants; very low-certainty evidence). Non-randomised studies of CA-125-targeted antibody therapy suggest improved survival among humoral and/or cellular responders, with only moderate adverse events. However, four large randomised placebo-controlled trials did not show any clinical benefit, despite induction of immune responses in approximately 60% of participants. Time to relapse with CA-125 monoclonal antibody versus placebo, respectively, ranged from 10.3 to 18.9 months versus 10.3 to 13 months (six RCTs, 1882 participants; high-certainty evidence). Only one RCT provided data on overall survival, reporting rates of 80% in both treatment and placebo groups (three RCTs, 1062 participants; high-certainty evidence). Other small studies targeting many different tumour antigens have presented promising immunological results. As these strategies have not yet been tested in RCTs, no reliable inferences about clinical efficacy can be made. Given the promising immunological results and the limited side effects and toxicity reported, exploration of clinical efficacy in large well-designed RCTs may be worthwhile.
Authors' conclusions: We conclude that despite promising immunological responses, no clinically effective antigen-specific active immunotherapy is yet available for ovarian cancer. Results should be interpreted cautiously, as review authors found a significant dearth of relevant information for assessment of risk of bias in both RCTs and NRSs.
Conflict of interest statement
Ninke Leffers, Cornelis Melief, Toos Daemen, and Hans Nijman were investigators in two studies included in this review (Leffers 2009a; Vermeij 2012). No potential conflicts of interest are known for the other contributing review authors (WH, BJC, STP, MDB) .
Figures
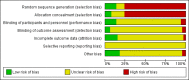
Update of
-
Antigen-specific active immunotherapy for ovarian cancer.Cochrane Database Syst Rev. 2014 Sep 17;(9):CD007287. doi: 10.1002/14651858.CD007287.pub3. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2018 Sep 10;9:CD007287. doi: 10.1002/14651858.CD007287.pub4. PMID: 25229990 Updated.
References
References to studies included in this review
Antonilli 2016 {published data only}
-
- Antonilli M, Rahimi H, Visconti V, Napoletano C, Ruscito I, Zizzari IG, et al. Triple peptide vaccination as consolidation treatment in women affected by ovarian and breast cancer: clinical and immunological data of a phase I/II clinical trial. International Journal of Oncology 2016;48:1369‐78. [PUBMED: 26892612] - PMC - PubMed
Baumann 2011 {published data only}
-
- Baumann K, Pfisterer J, Wimberger P, Burchardi P, Kurzeder C, du Bois A, et al. Intraperitoneal treatment with the trifunctional bispecific antibody catumaxomab in patients with platinum‐resistant epithelial ovarian cancer: a phase IIa study of the AGO Study Group. Gynecologic Oncology 2011;123:27‐32. - PubMed
Berek 2001 {published data only}
-
- Berek JS, Ehlen TG, Gordon A, Nicodemus CF, Schultes B, Whiteside TL, et al. Interim analysis of a double blind study of Ovared mAb B43.13 (OV) versus placebo (PBO) in patients with ovarian cancer. American Society of Clinical Oncology Annual Meeting. 2001.
Berek 2004 {published data only}
-
- Berek JS, Taylor PT, Gordon A, Cunningham MJ, Finkler N, Orr J, et al. Randomized, placebo‐controlled study of oregovomab for consolidation of clinical remission in patients with advanced ovarian cancer. Journal of Clinical Oncology 2004;22(17):3507‐16. - PubMed
-
- Berek JS, Taylor PT, Nicodemus CF. CA125 velocity at relapse is a highly significant predictor of survival post relapse: results of a 5‐year follow‐up survey to a randomized placebo‐controlled study of maintenance oregovomab immunotherapy in advanced ovarian cancer. Journal of Immunotherapy 2008;31(2):207‐14. - PubMed
Berek 2009 {published data only}
-
- Berek J, Taylor P, McGuire W, Smith LM, Schultes B, Nicodemus CF. Oregovomab maintenance monoimmunotherapy does not improve outcome in advanced ovarian cancer. Journal of Clinical Oncology 2009;27:418‐25. - PubMed
-
- Berek J, Taylor PT, McGuire WP, Smith LM, Schultes B, Nicodemus CF. Evaluation of maintenance mono‐immunotherapy to improve outcomes in advanced ovarian cancer (OV CA). ASCO Annual Meeting. 2008.
Berinstein 2012 {published data only}
-
- Berinstein NL, Karkada M, Morse MM, Nemunaitis JJ, Chatta G, Kaufman H, et al. First‐in‐man application of a novel therapeutic cancer vaccine formulation with the capacity to induce multi‐functional T cell responses in ovarian, breast and prostate cancer. Journal of Translational Medicine 2012;10:156. - PMC - PubMed
Berinstein 2013 {published data only}
-
- Berinstein NL, Oza AM, Odunsi K, Karkada M, Villella JA, Nemunaitis JJ, et al. Effect of oral cyclophosphamide on the immunogenicity of DPX‐Survivac in ovarian cancer patients: results of a phase I study. American Society of Clinical Oncology Annual Meeting. 2013.
Braly 2009 {published data only}
-
- Braly P, Chu C, Collins Y, Edwards R, Gordon A, McGuire W, et al. Prospective evaluation of front‐line chemo‐immunotherapy (C‐IT) with oregovomab (2 alternative dosing schedules) carboplatin‐paclitaxel (C‐P) in advanced ovarian cancer (OC). American Society of Clinical Oncology Annual Meeting. 2007.
-
- Braly P, Nicodemus CF, Chu C, Collins Y, Edwards R, Gordon A, et al. The immune adjuvant properties of front‐line carboplatin‐paclitaxel: a randomised phase 2 study of alternative schedules of intravenous oregovomab chemo‐immunotherapy in advanced ovarian cancer. Journal of Immunotherapy 2009;32:54‐65. - PubMed
-
- Method M, Gordon A, Smith LM, Nicodemus CF. Oregovomab immune‐modulating antibody therapy concurrent to standard chemotherapy of epithelial ovarian cancer (EOC): feasibility and initial clinical experience. Society of Gynecologic Oncologists Annual Meeting on Women's Cancer. 2006.
Brossart 2000 {published data only}
-
- Brossart P, Wirths S, Stuhler G, Reichardt VL, Kanz L, Brugger W. Induction of cytotoxic T‐lymphocyte responses in vivo after vaccinations with peptide‐pulsed dendritic cells. Blood 2000;96(9):3102‐8. - PubMed
Buzzonetti 2014 {published data only}
-
- Buzzonetti A, Fossati M, Catzola V, Scambia G, Fattorossi A, Battaglia A. Immunological response induced by abagovomab as a maintenance therapy in patients with epithelial ovarian cancer: relationship with survival ‐ a substudy of the MIMOSA trial. Cancer Immunology Immunotherapy 2014;63:1037‐45. [PUBMED: 24952307] - PMC - PubMed
Chianese‐Bullock 2008 {published data only}
-
- Chianese‐Bullock KA, Irvin WP, Petroni GR, Murphy C, Smolkin M, Olson WC, et al. A multipeptide vaccine is safe and elicits T‐cell responses in participants with advanced stage ovarian cancer. Journal of Immunotherapy 2008;31(4):420‐30. - PubMed
Chu 2012 {published data only}
-
- Chu CS, Boyer J, Coukos G, Rubin SC, Morgan MA, Bendig DL, et al. Autologous dendritic cell (IDD‐6) vaccination as consolidation for advanced ovarian cancer. Society of Gynecologic Oncologists Annual Meeting on Women's Cancer. 2008.
-
- Chu CS, Boyer J, Schullery DS, Gimotty PH, Gamerman V, Bender J, et al. Phase I/II randomized trial of dendritic cell vaccination with or without cyclophosphamide for consolidation therapy of advanced ovarian cancer in first or second remission. Cancer Immunology Immunotherapy 2012;61:629‐41. - PMC - PubMed
Dhodapkar 2012 {published data only}
-
- Dhodapkar M, Zhao B, Wang D, Lutzky RD, Carvajal RD, Keohan M, et al. A phase I trial of a novel vaccine targeting NY‐ESO‐1 to the dendritic cell receptor DEC‐205 in combination with toll‐like receptor agonists. Society for Immunotherapy of Cancer Annual Meeting. 2012.
Diefenbach 2008 {published data only}
-
- Diefenbach CSM, Gnjatic S, Sabbatini P, Aghajanian C, Hensley ML, Spriggs DR, et al. Safety and immunogenicity study of NY‐ESO‐1b peptide and montanide ISA‐51 vaccination of patients with epithelial ovarian cancer in high‐risk first remission. Clinical Cancer Research 2008;14(9):2740‐8. - PubMed
Dijkgraaf 2015 {published data only}
Ehlen 2005 {published data only}
-
- Ehlen TG, Hoskins PJ, Miller D, Whiteside TL, Nicodemus CF, Schultes BC, et al. A pilot phase 2 study of oregovomab murine monoclonal antibody to CA125 as an immunotherapeutic agent for recurrent ovarian cancer. International Journal of Gynecological Cancer 2005;15(6):1023‐34. - PubMed
Freedman 1998 {published data only}
-
- Freedman RS, Kudelka AP, Verschraegen CF, Edwards CL, Tomasovic B, Kaplan A, et al. Therapeutic anti‐cancer vaccine: a randomized double blind dose comparison study of sialyl Tn‐KLH with Detox‐B SE adjuvant for active specific immunotherapy of ovarian cancer (OC). American Society of Clinical Oncology Annual Meeting. 1998.
-
- Termrungruanglert W, Kudelka AP, Verschraegen CF, Freedman RS, Edwards CL, Tomasovic B, et al. Therapeutic anticancer vaccine: a randomized double blind dose comparison study of sialyl TN‐K with detox‐B SE adjuvant for active specific immunotherapy of ovarian cancer (OC). American Society of Clinical Oncology Annual Meeting. 1996.
Galanis 2010 {published data only}
Goh 2013 {published data only}
-
- Goh J, CAN‐003 Study Team, Gargosky SE, Gray H. Clinical study of autologous dendritic cell therapy targeting mucin 1 for treatment of ovarian cancer patients in first remission. European Cancer Congress. 2013.
Gordon 2004 {published data only}
-
- Gordon AN, Schultes BC, Gallion H, Edwards R, Whiteside TL, Cermak JM, et al. CA125‐ and tumor‐specific T‐cell responses correlate with prolonged survival in oregovomab‐treated recurrent ovarian cancer patients. Gynecologic Oncology 2004;94(2):340‐51. - PubMed
Gray 2016 {published data only}
Gribben 2005 {published data only}
-
- Gribben JG, Ryan DP, Urban RG, Hedley ML, Beach K, Nealon P, et al. Unexpected association between induction of immunity to the universal tumor antigen CYP1B1 and response to next therapy. Clinical Cancer Research 2005;11(12):4430‐6. - PubMed
Gulley 2008 {published data only}
Heiss 2010 {published data only}
-
- Heiss MM, Maruwa P, Koralewski P, Kutarska E, Kolesnik OO, Ivanchenko VV, et al. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: results of a prospective randomized phase II/III trial. International Journal of Cancer 2010;127:2209‐21. - PMC - PubMed
Imhof 2013 {published data only}
-
- Imhoff M, Lipovac M, Angleitner‐Boubenizek L, Barta J, Gomez I, Hrdina A, et al. Double‐loaded mature dendritic cell (DC) therapy for non‐HLA‐restricted patients with advanced ovarian cancer: final results of a clinical phase I study. American Society of Clinical Oncolocy Annual Meeting. 2013.
Kaumaya 2009 {published data only}
-
- Kaumaya PTP, Foy KC, Garrett J, Rawale SV, Vicari D, Thurmond JM, et al. Phase I active immunotherapy with combination of two chimeric, human epidermal growth factor receptor 2, b‐cell epitopes fused to a promiscuous T‐cell epitope in patients with metastatic and/or recurrent solid tumors. Journal of Clinical Oncology 2009;27(31):5270‐7. - PMC - PubMed
Kawano 2014 {published data only}
-
- Kawano K, Tsuda N, Matsueda S, Sasada T, Watanabe N, Ushijima K, et al. Feasibility study of personalized peptide vaccination for recurrent ovarian cancer patients. Immunopharmacology Immunotoxicology 2014;36:224‐36. [PUBMED: 24773550] - PubMed
Kobayashi 2014 {published data only}
Le 2012 {published data only}
-
- Le DT, Brockstedt DG, Nir‐Paz R, Hampl J, Mathur S, Nemunaitis J, et al. A live‐attenuated listeria vaccine (ANZ‐100) and a live‐attenuated listeria vaccine expressing mesothelin (CRS‐207) for advanced cancers: phase I studies of safety and immune induction. Clinical Cancer Research 2012;18(3):858‐68. - PMC - PubMed
Leffers 2009a {published data only}
-
- Leffers N, Lambeck AJ, Gooden MJ, Hoogeboom BN, Wolf R, Hamming IE, et al. Immunization with a P53 synthetic long peptide vaccine induces P53‐specific immune responses in ovarian cancer patients, a phase II trial. International Journal of Cancer 2009;May 28:[Epub ahead of print]. - PubMed
-
- Leffers N, Vermeij R, Hoogeboom BN, Schultze UR, Wolf R, Hamming IE, et al. Long‐term clinical and immunological effects of p53‐SLP vaccine in patients with ovarian cancer. International Journal of Cancer 2012;130:105‐12. - PubMed
Lennerz 2014 {published data only}
Letsch 2011 {published data only}
-
- Letsch A, Scheibenbogen C, Asemissen AM, Zimmermann K, Knodler M, Völker‐Call M, et al. Wilms tumor protein (WT) 1 peptide vaccination in patients with WT1 expressing solid tumors demonstrates clinical and immunological efficacy. Onkologie 2011;34(Suppl 6):194.
Ma 2002 {published data only}
MacLean 1992 {published data only}
-
- MacLean GD, Bowen‐Yacyshyn MB, Samuel J, Meikle A, Stuart G, Nation J, et al. Active immunization of human ovarian cancer patients against a common carcinoma (Thomsen‐Friedenreich) determinant using a synthetic carbohydrate antigen. Journal of Immunotherapy 1992;11(4):292‐305. - PubMed
MacLean 1996 {published data only}
-
- MacLean GD, Reddish MA, Koganty RR, Longenecker BM. Antibodies against mucin‐associated sialyl‐Tn epitopes correlate with survival of metastatic adenocarcinoma patients undergoing active specific immunotherapy with synthetic STn vaccine. Journal of Immunotherapy With Emphasis on Tumor Immunology 1996;19(1):59‐68. - PubMed
Method 2002 {published data only}
-
- Method M, Gordon A, Finkler N, Fingert H, Nicodemus CF, Whiteside TL, et al. Randomized evaluation of 3 treatment schedules to optimize clinical activity of OvaRex Mab B43.13 (OV) in patients (pts) with epithelial ovarian cancer (EOC). American Society of Clinical Oncology Annual Meeting. 2002.
Möbus 2003 {published data only}
-
- Mobus VJ, Baum RP, Bolle M, Kreienberg R, Noujaim AA, Schultes BC, et al. Immune responses to murine monoclonal antibody‐B43.13 correlate with prolonged survival of women with recurrent ovarian cancer. American Journal of Obstetrics and Gynecology 2003;189(1):28‐36. - PubMed
Mohebtash 2011 {published data only}
-
- Mohebtash M, Madan R, Tsang K, Arlen Ph, Pazdur M, Jones J, et al. Clinical outcomes following immunotherapy with a MUC1/CEA vaccine in patients with metastatic breast and ovarian cancer. American Association of Cancer Research Annual Meeting. 2009.
Morse 2011 {published data only}
-
- Morse MA, Secord AA, Blackwell K, Hobeika AM, Sinnathamby G, Osada T, et al. MHC class I‐presented tumor antigens identified in ovarian cancer by immunoproteomic analysis are targets for T‐cell responses against breast and ovarian cancer. Clinical Cancer Research 2011;17(10):3408‐19. - PubMed
Nicholson 2004 {published data only}
Nishikawa 2006 {published data only}
-
- Nishikawa H, Qian F, Tsuji T, Ritter G, Old LJ, Gnjatic S, et al. Influence of CD4(+)CD25(+) regulatory T cells on low/high‐avidity CD4(+) T cells following peptide vaccination. Journal of Immunology 2006;176(10):6340‐6. - PubMed
Noujaim 2001 {published data only}
-
- Noujaim AA, Schultes BC, Baum RP, Madiyalakan R. Induction of CA125‐specific B and T cell responses in patients injected with MAb‐B43.13 ‐ evidence for antibody‐mediated antigen‐processing and presentation of CA125 in vivo. Cancer Biotherapy & Radiopharmaceuticals 2001;16(3):187‐203. - PubMed
O'Cearbhaill 2016 {published data only}
Odunsi 2007 {published data only}
-
- Odunsi K, Qian F, Matsuzaki J, Mhawech‐Fauceglia P, Andrews C, Hoffman EW, et al. Vaccination with an NY‐ESO‐1 peptide of HLA class I/II specificities induces integrated humoral and T cell responses in ovarian cancer. Proceedings of the National Academy of Sciences of the United States of America 2007;104(31):12837‐42. - PMC - PubMed
Odunsi 2012 {published data only}
-
- Odunsi K, Rodabaugh K, Lele S, Old LJ, Matsuzaki J, Qian F, et al. Diversified prime and boost vaccination using recombinant vaccinia and fowlpox expressing NY‐ESO‐1 efficiently induces antibody, CD4+, and CD8+ antitumor immune responses in patients with ovarian cancer. Society of Gynecologic Oncologists Annual Meeting on Women's Cancer. 2007.
Odunsi 2014 {published data only}
Ohno 2009 {published data only}
-
- Dohi S, Ohno S, Ohno Y, Takakura M, Kyo S, Soma GI, et al. WT1 peptide vaccine stabilized intractable ovarian cancer patient for one year: a case report. Anticancer Research 2001;31:2441‐6. - PubMed
-
- Ohno S, Kyo S, Myojo S, Dohi S, Ishizaki J, Miyamoto KI, et al. Wilms' tumor 1 (WT1) peptide immunotherapy for gynecological malignancy. Anticancer Research 2009;29:4779‐84. - PubMed
Peethambaram 2009 {published data only}
Pfisterer 2006 {published data only}
-
- Pfisterer J, du Bois A, Sehouli J, Loibl S, Reinartz S, Reuss A, et al. The anti‐idiotypic antibody abagovomab in patients with recurrent ovarian cancer. A phase I trial of the AGO‐OVAR. Annals of Oncology 2006;17(10):1568‐77. - PubMed
Rahma 2012 {published data only}
-
- Herrin V, Achtar M, Steinberg S, Whiteside TL, Wieckowsk E, Czystowska M, et al. A randomized phase II p53 vaccine trial comparing subcutaneous direct administration with intravenous peptide‐pulsed dendritic cells in high risk ovarian cancer patients. American Society of Clinical Oncology Annual Meeting. 2007.
-
- Herrin V, Behrens RJ, Achtar M, Monahan B, Bernstein S, Brent‐Steele T, et al. Wild‐type p53 peptide vaccine can generate a specific immune response in low burden ovarian adenocarcinom. American Society of Clinical Oncology Annual Meeting. 2003.
-
- Rahma OE, Ashtar E, Czystowska M, Szajnik ME, Wieckowski E, Bernstein S, et al. A gynecologic oncology group phase II trial of two p53 peptide vaccine approaches: subcutaneous injection and intravenous pulsed dendritic cells in high recurrence risk ovarian cancer patients. Cancer Immunology, Immunotherapy 2012;61:374‐84. - PMC - PubMed
Reinartz 2004 {published data only}
-
- Reinartz S, Kohler S, Schlebusch H, Krista K, Giffels P, Renke K, et al. Vaccination of patients with advanced ovarian carcinoma with the anti‐idiotype ACA125: immunological response and survival (phase Ib/II). Clinical Cancer Research 2004;10(5):1580‐7. - PubMed
-
- Wagner U, Kohler S, Reinartz S, Giffels P, Huober J, Renke K, et al. Immunological consolidation of ovarian carcinoma recurrences with monoclonal anti‐idiotype antibody ACA125: immune responses and survival in palliative treatment. Clinical Cancer Research 2001;7(5):1154‐62. - PubMed
Sabbatini 2000 {published data only}
-
- Sabbatini PJ, Kudryashov V, Ragupathi G, Danishefsky SJ, Livingston PO, Bornmann W, et al. Immunization of ovarian cancer patients with a synthetic Lewis(y)‐protein conjugate vaccine: a phase 1 trial. Clinical Cancer Research 2000;87(1):79‐85. - PubMed
Sabbatini 2006 {published data only}
-
- Sabbatini P, Dupont J, Aghajanian C, Derosa F, Poynor E, Anderson S, et al. Phase I study of abagovomab in patients with epithelial ovarian, fallopian tube, or primary peritoneal cancer. Clinical Cancer Research 2006;12(18):5503‐10. - PubMed
Sabbatini 2007 {published data only}
-
- Sabbatini PJ, Ragupathi G, Hood C, Aghajanian CA, Juretzka M, Iasonos A, et al. Pilot study of a heptavalent vaccine‐keyhole limpet hemocyanin conjugate plus QS21 in patients with epithelial ovarian, fallopian tube, or peritoneal cancer. Clinical Cancer Research 2007;13:4170‐7. - PubMed
Sabbatini 2012 {published data only}
-
- Sabbatini P, Tsuji T, Ferran L, Ritter E, Sedrak C, Tuballes K, et al. Phase I trial of overlapping long peptides from a tumor self‐antigen and poly‐ICLC shows rapid induction of integrated immune response in ovarian cancer patients. Clinical Cancer Research 2012;18(23):6497‐508. - PubMed
Sabbatini 2013 {published data only}
Sabbatini 2017 {published data only}
-
- Sabbatini P, Chen L, Lucci J, Behbakht K, Spirtos N, Muller C. OPT‐821 with or without vaccine therapy in treating patients with ovarian epithelial cancer, fallopian tube cancer, or peritoneal cancer in second or third complete remission. clinicaltrials.gov 2017. [NCT00857545]
Sandmaier 1999 {published data only}
-
- Sandmaier BM, Oparin DV, Holmberg LA, Reddish MA, MacLean GD, Longenecker BM. Evidence of a cellular immune response against sialyl‐Tn in breast and ovarian cancer patients after high‐dose chemotherapy, stem cell rescue, and immunization with Theratope STn‐KLH cancer vaccine. Journal of Immunotherapy 1999;22(1):54‐66. - PubMed
Schultes 1998 {published data only}
-
- Schultes B, Yang R, Agopsowicz K, Kuzma M, Dharampaul S, Baum R, et al. Anti‐idiotype induction therapy for ovarian cancer: immune responses in patients injected with OvaRex‐Mab‐B43.13. American Society of Clinical Oncology Annual Meeting. 1999.
Ströhlein 2009 {published data only}
Suzuki 2016 {published data only}
Takeoka 2017 {published data only}
-
- Takeoka T, Nagase H, Kurose K, Ohue Y, Yamasaki M, Takiguchi S, et al. NY‐ESO‐1 protein cancer vaccine with Poly‐ICLC and OK‐432: rapid and strong induction of NY‐ESO‐1‐specific immune responses by Poly‐ICLC. Journal of Immunotherapy 2017;40:140‐7. [PUBMED: 28338507] - PubMed
Takeuchi 2013 {published data only}
-
- Takeuchi S, Shoji T, Kagabu M, Honda T, Miura F, Omi H, et al. A phase I/II study of multiple peptides cocktail vaccine for advanced/recurrent ovarian cancer. American Society of Clinical Oncology Annual Meeting. 2013.
Tsuda 2004 {published data only}
-
- Tsuda N, Mochizuki K, Harada M, Sukehiro A, Kawano K, Yamada A, et al. Vaccination with predesignated or evidence‐based peptides for patients with recurrent gynecologic cancers. Journal of Immunotherapy 2004;27(1):60‐72. - PubMed
van Zanten‐Przybysz 2002 {published data only}
-
- Zanten‐Przybysz I, Molthoff C, Gebbinck JK, Mensdorff‐Pouilly S, Verstraeten R, Kenemans P, et al. Cellular and humoral responses after multiple injections of unconjugated chimeric monoclonal antibody MOv18 in ovarian cancer patients: a pilot study. Journal of Cancer Research and Clinical Oncology 2002;128(9):484‐92. - PMC - PubMed
Vermeij 2012 {published data only}
-
- Vermeij R, Leffers N, Hoogeboom BN, Hamming LE, Wolf R, Reyners AKL, et al. Potentiation of a p53‐SLP vaccine by cyclophosphamide in ovarian cancer: a single‐arm phase II study. International Journal of Cancer 2012;131:E670‐80. - PubMed
Wagner 1993 {published data only}
-
- Wagner U. Antitumor antibodies for immunotherapy of ovarian carcinomas. Hybridoma 1993;12(5):521‐8. - PubMed
-
- Wagner U, Reinsberg J, Schmidt S, Mallmann P, Schmolling J, Schultes B, et al. Monoclonal antibodies and idiotypic network activation for ovarian carcinoma. Cell Biophysics 1994;24‐25:237‐42. - PubMed
References to studies excluded from this review
Anderson 2000 {published data only}
Baek 2015 {published data only}
Bapsy 2014 {published data only}
-
- Bapsy PP, Sharan B, Kumar C, Das RP, Rangarajan B, Jain M. Open‐label, multi‐center, non‐randomized, single‐arm study to evaluate the safety and efficacy of dendritic cell immunotherapy in patients with refractory solid malignancies, on supportive care. Cytotherapy 2014;16(2):234‐44. [PUBMED: 24438902] - PubMed
Bender 2007 {published data only}
Bernal 2012 {published data only}
Carbone 2005 {published data only}
-
- Carbone DP, Ciernik IF, Kelley MJ, Smith MC, Nadaf S, Kavanaugh D, et al. Immunization with mutant p53‐ and K‐ras‐derived peptides in cancer patients: immune response and clinical outcome. Journal of Clinical Oncology 2005;23(22):5099‐107. - PubMed
Chiang 2013 {published data only}
-
- Chiang CL, Kandalaft LE, Tanyi J, Hagemann AR, Motz GT, Svoronos N. A dendritic cell vaccine pulsed with autologous hypochlorous acid‐oxidized ovarian cancer lysate primes effective broad antitumor immunity: from bench to bedside. Clinical Cancer Research 2013;19(17):4801‐15. [PUBMED: 23838316] - PMC - PubMed
Coosemans 2013 {published data only}
-
- Coosemans A, Vanderstraeten A, Tuyaerts S, Verschuere T, Moerman P, Berneman Z. Immunological response after WT1 mRNA‐loaded dendritic cell immunotherapy in ovarian carcinoma and carcinosarcoma. Anticancer Research 2013;33(9):3855‐9. [PUBMED: 24023319] - PubMed
Dhodapkar 2014 {published data only}
Disis 1999 {published data only}
-
- Disis ML, Grabstein KH, Sleath PR, Cheever MA. Generation of immunity to the HER‐2/neu oncogenic protein in patients with breast and ovarian cancer using a peptide‐based vaccine. Clinical Cancer Research 1999;5(6):1289‐97. - PubMed
Disis 2000 {published data only}
-
- Disis ML, Schiffman K, Gooley TA, McNeel DG, Rinn K, Knutson KL. Delayed‐type hypersensitivity response is a predictor of peripheral blood T‐cell immunity after HER‐2/neu peptide immunization. Clinical Cancer Research 2000;6(4):1347‐50. - PubMed
Disis 2002 {published data only}
-
- Disis ML, Gooley TA, Rinn K, Davis D, Piepkorn M, Cheever MA, et al. Generation of T‐cell immunity to the HER‐2/neu protein after active immunization with HER‐2/neu peptide‐based vaccines. Journal of Clinical Oncology 2002;20(11):2624‐32. - PubMed
Disis 2002a {published data only}
-
- Disis ML, Rinn K, Knutson KL, Davis D, Caron D, dela Rosa C, et al. Flt3 ligand as a vaccine adjuvant in association with HER‐2/neu peptide‐based vaccines in patients with HER‐2/neu‐overexpressing cancers. Blood 2002;99(8):2845‐50. - PubMed
Disis 2004 {published data only}
-
- Disis ML, Goodell V, Schiffman K, Knutson KL. Humoral epitope‐spreading following immunization with a HER‐2/neu peptide based vaccine in cancer patients. Journal of Clinical Immunology 2004;24(5):571‐8. - PubMed
Disis 2004a {published data only}
-
- Disis ML, Schiffman K, Guthrie K, Salazar LG, Knutson KL, Goodell V, et al. Effect of dose on immune response in patients vaccinated with an her‐2/neu intracellular domain protein‐‐based vaccine. Journal of Clinical Oncology 2004;22(10):1916‐25. - PubMed
Galanis 2013 {published data only}
-
- Galanis E, Atherton P, Dowdy S, Cliby W, Haluska P, Long H, et al. Intraperitoneal (IP) administration of an oncolytic measles virus (MV) strain expressing the sodium iodine symporter gene in patients (pts) with advanced ovarian cancer (ovca). American Society of Gene & Cell Therapy (ASGCT) Annual Meeting. 2013.
Haakenstad 2012 {published data only}
-
- Haakenstad H, Suso EMI, Rasmussen AM, Larsen SS, Dueland S, Lilleby W, et al. Clinical use of fast DCs transfected with hTERT and Survivin mRNA ‐ an effective and simplified cancer vaccine approach. European Group for Blood and Marrow Transplantation Annual Meeting. 2012.
-
- Kvalheim G, Suso E, Rasmussen A, Honnashagen T, Dueland S, Gaudernack G. Fast DCs transfected with hTERT and Survivin mRNA ‐ a novel, effective and simplified cancer vaccine approach. European Group for Blood and Marrow Transplantation Annual Meeting. 2011.
Hasumi 2011 {published data only}
Hernando 2002 {published data only}
-
- Hernando JJ, Park TW, Kubler K, Offergeld R, Schlebusch H, Bauknecht T. Vaccination with autologous tumour antigen‐pulsed dendritic cells in advanced gynaecological malignancies: clinical and immunological evaluation of a phase I trial. Cancer Immunology, Immunotherapy 2002;51(1):45‐52. - PMC - PubMed
Hernando 2007 {published data only}
-
- Hernando JJ, Park T‐W, Fischer H‐P, Zivanovic O, Braun M, Polcher M, et al. Vaccination with dendritic cells transfected with mRNA‐encoded folate‐receptor‐(alpha) for relapsed metastatic ovarian cancer. Lancet Oncology 2007;8(5):451‐4. - PubMed
Holmberg 2000 {published data only}
-
- Holmberg LA, Oparin DV, Gooley T, Lilleby K, Bensinger W, Reddish MA, et al. Clinical outcome of breast and ovarian cancer patients treated with high‐dose chemotherapy, autologous stem cell rescue and THERATOPE STn‐KLH cancer vaccine. Bone Marrow Transplant 2000;25(12):1233‐41. - PubMed
Hui 1997 {published data only}
-
- Hui KM, Ang PT, Huang L, Tay SK. Phase I study of immunotherapy of cutaneous metastases of human carcinoma using allogeneic and xenogeneic MHC DNA‐liposome complexes. Gene Therapy 1997;4(8):783‐90. - PubMed
Jackson 2017 {published data only}
Jager 2006 {published data only}
-
- Jager E, Karbach J, Gnjatic S, Neumann A, Bender A, Valmori D, et al. Recombinant vaccinia/fowlpox NY‐ESO‐1 vaccines induce both humoral and cellular NY‐ESO‐1‐specific immune responses in cancer patients. Proceedings of the National Academy of Sciences of the United States of America 2006;103(39):14453‐8. - PMC - PubMed
Kandalaft 2010 {published data only}
-
- Coukos G, Powell D, Kandalaft L, Smith L, Chu C, Rubin S, et al. Autologous whole‐tumor antigen combinatorial immunotherapy for recurrent ovarian cancer. Gynecologic Oncology 2010;116:s130.
-
- Kandalaft LE, Powell DJ, Smith L, Adams S, Liao J, Hageman A, et al. Autologous whole‐tumor anitgen combinatorial immunotherapy for recurrent ovarian cancer. Journal of Immunotherapy 2010;33(8):878.
Karbach 2010 {published data only}
-
- Karbach J, Gnjatic S, Bender A, Neumann A, Weidmann E, Yuan J, et al. Tumor‐reactive CD8+ T‐cell responses after vaccination with NY‐ESO‐1 peptide, CpG 7909 and Montanide ISA‐51: association with survival. International Journal of Cancer 2010;126:909‐18. - PubMed
Kato 2010 {published data only}
-
- Kato Y. WT1 peptide pulsed dendritic cell therapy with activated T lymphocytes therapy for advanced cancers. Gan To Kagaku Ryoho 2010;37(12):2240‐2. - PubMed
Khranovska 2011 {published data only}
-
- Khranovska NM, Svyntsytsky VS, Potebnya GP, Vorobyova LI, Skachkova OV, Tsyp NP, et al. New dendritic cell vaccine therapy approach ‐ randomized phase I/II study in III‐IV stage ovarian cancer patients. European Multidisciplinary Cancer Conference (ECCO ESMO ESTRO). 2011.
Knutson 2001 {published data only}
Knutson 2002 {published data only}
-
- Knutson KL, Schiffman K, Cheever MA, Disis ML. Immunization of cancer patients with a HER‐2/neu, HLA‐A2 peptide, p369‐377, results in short‐lived peptide‐specific immunity. Clinical Cancer Research 2002;8(5):1014‐8. - PubMed
Letsch 2008 {published data only}
-
- Letsch A, Asemissen AM, Zimmermann K, Bauer S, Stather D, Völker‐Call M, et al. Different quality of T cell responses to WT1 peptide vaccination in patients with AML/MDS and patients with solid tumors. Journal of Immunotherapy. 2008; Vol. 31:943‐4.
Loveland 2006 {published data only}
-
- Loveland BE, Zhao A, White S, Gan H, Hamilton K, Xing PX, et al. Mannan‐MUC1‐pulsed dendritic cell immunotherapy: a phase I trial in patients with adenocarcinoma. Clinical Cancer Research 2006;12(3 Pt 1):869‐77. - PubMed
Manjunath 2012 {published data only}
Marshall 2005 {published data only}
-
- Marshall JL, Gulley JL, Arlen PM, Beetham PK, Tsang KY, Slack R, et al. Phase I study of sequential vaccinations with fowlpox‐CEA(6D)‐TRICOM alone and sequentially with vaccinia‐CEA(6D)‐TRICOM, with and without granulocyte‐macrophage colony‐stimulating factor, in patients with carcinoembryonic antigen‐expressing carcinomas. Journal of Clinical Oncology 2005;23:720‐31. - PubMed
Matsuzaki 2014 {published data only}
Miotti 1999 {published data only}
-
- Miotti S, Negri DR, Valota O, Calabrese M, Bolhuis RL, Gratama JW, et al. Level of anti‐mouse‐antibody response induced by bi‐specific monoclonal antibody OC/TR in ovarian‐carcinoma patients is associated with longer survival. International Journal of Cancer 1999;84(1):62‐8. - PubMed
Morera 2017 {published data only}
-
- Morera Y, Sánchez J, Bequet‐Romero M, Selman‐Housein KH, Torre A, Hernández‐Bernal F. Specific humoral and cellular immune responses in cancer patients undergoing chronic immunization with a VEGF‐based therapeutic vaccine. Vaccine 2017;35:3582‐90. [PUBMED: 28536029] - PubMed
Morse 1999 {published data only}
-
- Morse MA, Deng Y, Coleman D, Hull S, Kitrell‐Fisher E, Nair S, et al. A Phase I study of active immunotherapy with carcinoembryonic antigen peptide (CAP‐1)‐pulsed, autologous human cultured dendritic cells in patients with metastatic malignancies expressing carcinoembryonic antigen. Clinical Cancer Research 1999;5(6):1331‐8. - PubMed
Morse 2003 {published data only}
-
- Morse MA, Clay TM, Colling K, Hobeika A, Grabstein K, Cheever MA, et al. Her2 dendritic cell vaccines. Clinical Breast Cancer 2003;3(Suppl 4):S164‐72. - PubMed
Morse 2011a {published data only}
Murray 2002 {published data only}
-
- Murray JL, Gillogly ME, Przepiorka D, Brewer H, Ibrahim NK, Booser DJ, et al. Toxicity, immunogenicity, and induction of E75‐specific tumor‐lytic CTLs by HER‐2 peptide E75 (369‐377) combined with granulocyte macrophage colony‐stimulating factor in HLA‐A2+ patients with metastatic breast and ovarian cancer. Clinical Cancer Research 2002;8(11):3407‐18. - PubMed
Oh 2016 {published data only}
-
- Oh J, Barve M, Matthews CM, Koon EC, Heffernan TP, Fine B. Phase II study of Vigil® DNA engineered immunotherapy as maintenance in advanced stage ovarian cancer. Gynaecologic Oncology 2016;143(3):504‐10. [27678295] - PubMed
Parkhurst 2004 {published data only}
Reddish 1996 {published data only}
Salazar 2006 {published data only}
-
- Salazar LG, Murray JL, Disis ML, Cheever M. A phase I vaccine trial of a HER‐2/neu peptide incorporated into PLG microspheres in patients with advanced stage HER2‐expressing cancers. ASCO Annual Meeting. 2006.
Schiffman 2002 {published data only}
-
- Schiffman K, Rinn K, Disis ML. Delayed type hypersensitivity response to recall antigens does not accurately reflect immune competence in advanced stage breast cancer patients. Breast Cancer Research and Treatment 2002;74(1):17‐23. - PubMed
Tsuji 2013 {published data only}
-
- Tsuji T, Sabbatini P, Jungbluth AA, Ritter E, Pan L, Ritter G, et al. Effect of Montanide and poly‐ICLC adjuvant on human self/tumor antigen‐specific CD4+ T cells in phase I overlapping long peptide vaccine trial. Cancer Immunology Research 2013;1:340‐50. [DOI: 10.1158/2326-6066.CIR-13-0089] - DOI - PubMed
Yacyshyn 1995 {published data only}
-
- Yacyshyn MB, Poppema S, Berg A, MacLean GD, Reddish MA, Meikle A, et al. CD69+ and HLA‐DR+ activation antigens on peripheral blood lymphocyte populations in metastatic breast and ovarian cancer patients: correlations with survival following active specific immunotherapy. International Journal of Cancer 1995;61(4):470‐4. - PubMed
Zaks 1998 {published data only}
-
- Zaks TZ, Rosenberg SA. Immunization with a peptide epitope (p369‐377) from HER‐2/neu leads to peptide‐specific cytotoxic T lymphocytes that fail to recognize HER‐2/neu+ tumors. Cancer Research 1998;58(21):4902‐8. - PubMed
References to ongoing studies
NCT00003002 {unpublished data only}
-
- University of Washington. Her‐2/Neu vaccine plus GM‐CSF in treating patients with stage III or stage IV breast, ovarian, or non‐small cell lung cancer. clinicaltrials.gov.
NCT00004604 {unpublished data only}
-
- Duke University. Biological therapy in treating patients with metastatic cancer. clinicaltrials.gov.
NCT00006041 {unpublished data only}
-
- Memorial Sloan ‐ Kettering Cancer Center. Vaccine therapy in treating patients with ovarian, fallopian tube, or peritoneal cancer. clinicaltrials.gov.
NCT00381173 {unpublished data only}
-
- Eisai Medical Research Inc. A phase 1 open‐label study of the safety and feasibility of ZYC300 administration with cyclophosphamide pre‐dosing. clinicaltrials.gov.
NCT00803569 {unpublished data only}
-
- Roswell Park Cancer Institute and National Cancer Institute (NCI). Phase I study of ALVAC(2)‐NY‐ESO‐1(M)/TRICOM in patients with epithelial ovarian, fallopian tube or primary peritoneal carcinoma whose tumors express NY‐ESO‐1 or LAGE‐1 antigen. clinicaltrilas.gov.
NCT01223235 {unpublished data only}
-
- Memorial Sloan ‐ Kettering Cancer Center. Polyvalent vaccine‐KLH conjugate + Opt‐821 given in combination with bevacuzimab. clinicaltrials.gov.
NCT01322802 {unpublished data only}
-
- University of Washington. Vaccine therapy in treating patients with stage III‐IV or recurrent ovarian cancer. clinicaltrials.gov.
NCT01376505 {unpublished data only}
-
- Pravin Kaumaya. Vaccine therapy in treating patients with metastatic solid tumors. clinicaltrials.gov.
NCT01522820 {unpublished data only}
-
- Roswell Park Cancer Institute. Vaccine therapy with or without sirolimus in treating patients with NY‐ESO‐1 expressing solid tumors. clinicaltrials.gov.
NCT01536054 {unpublished data only}
-
- Roswell Park Cancer Institute. Sirolimus and vaccine therapy in treating patients with stage II‐IV ovarian epithelial, fallopian tube, or primary peritoneal cavity cancer. clinicaltrials.gov.
NCT01556841 {unpublished data only}
-
- Oxford BioMedica. The activity of TroVax versus placebo in relapsed asymptomatic ovarian cancer (TRIOC). clinicaltrials.gov.
NCT01584115 {unpublished data only}
-
- Instituto de Investigacao em Imunologia. Clinical trial of a therapeutic vaccine with NY‐ESO‐1 in combination with the adjuvant monophosphoryl lipid A (MPLA). clinicaltrials.gov.
NCT01606241 {unpublished data only}
-
- Mayo Clinic. Cyclophosphamide and vaccine therapy in treating patients with stage II‐III breast, ovarian, primary peritoneal, or fallopian tube cancer. clinicaltrials.gov.
NCT01616303 {unpublished data only}
-
- Quest PharmaTech Inc. A controlled study of the effectiveness of oregovomab (antibody) plus chemotherapy in advanced ovarian cancer. clinicaltrials.gov.
NCT01621542 {unpublished data only}
-
- Sunovion. Clinical study of WT2725 in patients with advanced solid malignancies. clinicaltrials.gov.
NCT01673217 {unpublished data only}
-
- Roswell Park Cancer Institute. Decitabine, vaccine therapy, and pegylated liposomal doxorubicin hydrochloride in treating patients with recurrent ovarian epithelial cancer, fallopian tube cancer, or peritoneal cancer. clinicaltrials.gov.
NCT02111941 {unpublished data only}
-
- Mayo Clinic. Vaccine therapy in treating patients with stage IIIC‐IV ovarian epithelial, fallopian tube, or primary peritoneal cavity cancer following surgery and chemotherapy. clinicaltrials.gov.
NCT02132988 {unpublished data only}
-
- Mackay Memorial Hospital. Trial of active immunotherapy with Globo H‐KLH (OPT‐822/821) in women who have non‐progressive ovarian cancer. clinicaltrials.gov.
NCT02146313 {unpublished data only}
-
- Genentech, Inc. A study evaluating the safety and pharmacokinetics of DMUC4064A in participants with platinum‐resistant ovarian cancer or unresectable pancreatic cancer. clinicaltrials.gov.
NCT02166905 {unpublished data only}
-
- Roswell Park Cancer Institute. DEC‐205/NY‐ESO‐1 fusion protein CDX‐1401, Poly ICLC, and IDO1 inhibitor INCB024360 in treating patients with ovarian, fallopian tube, or primary peritoneal cancer in remission. clinicaltrials.gov.
NCT02275039 {unpublished data only}
-
- City of Hope Medical Center. P53MVA vaccine and gemcitabine hydrochloride in treating patients with recurrent ovarian epithelial cancer. clinicaltrials.gov.
NCT02387125 {unpublished data only}
-
- Immune Design. Phase 1b safety study of CMB305 in patients with locally advanced, relapsed, or metastatic cancer expressing NY‐ESO‐1. clinicaltrials.gov. - PMC - PubMed
NCT02498665 {unpublished data only}
-
- Boston Biomedical, Inc. A study of DSP‐7888 dosing emulsion in adult patients with advanced malignancies. clinicaltrials.gov.
NCT02575807 {unpublished data only}
-
- Aduro Biotech, Inc. Safety and efficacy of CRS‐207 with Epacadostat in platinum resistant ovarian, fallopian or peritoneal cancer (SEASCAPE). clinicaltrials.gov.
NCT02737787 {unpublished data only}
-
- Memorial Sloan Kettering Cancer Center. A study of WT1 vaccine and nivolumab for recurrent ovarian cancer. clinicaltrials.gov.
NCT02764333 {unpublished data only}
-
- Memorial Sloan Kettering Cancer Center. TPIV200/huFR‐1 (a multi‐epitope anti‐folate receptor vaccine) plus anti‐PD‐L1 MEDI4736 (Durvalumab) in patients with platinum resistant ovarian cancer. clinicaltrials.gov.
NCT02785250 {unpublished data only}
-
- ImmunoVaccine Technologies, Inc. Study of DPX‐Survivac vaccine therapy and Epacadostat in patients with recurrent ovarian cancer. clinicaltrials.gov.
NCT02833506 {unpublished data only}
-
- Roswell Park Cancer Institute. Sirolimus and vaccine therapy in treating patients with stage II‐IV ovarian, fallopian tube, or primary peritoneal cancer. clinicaltrials.gov.
NCT02933073 {unpublished data only}
-
- UConn Health. Study of oncoimmunome for the treatment of stage III/IV ovarian carcinoma. clinicaltrials.gov.
NCT02978222 {unpublished data only}
-
- Tapimmune Inc. Folate receptor alpha peptide vaccine with GM‐CSF versus GM‐CSF alone in patients with platinum sensitive ovarian cancer. Clinicaltrials.gov.
NCT03029403 {unpublished data only}
-
- University Health Network, Toronto. Phase 2 study of Pembrolizumab, DPX‐Survivac vaccine and Cyclophosphamide in advanced ovarian, primary peritoneal or fallopian tube cancer. clinicaltrials.gov.
NCT03029611 {unpublished data only}
-
- University of Washington. IGFBP‐2 vaccine and combination chemotherapy in treating patients with stage III‐IV ovarian, fallopian tube, or primary peritoneal cancer undergoing surgery. clinicaltrials.gov.
NCT03113487 {unpublished data only}
-
- City of Hope Medical Center. P53MVA and Pembrolizumab in treating patients with recurrent ovarian, primary peritoneal, or fallopian tube cancer. clinicaltrials.gov.
NCT03127098 {unpublished data only}
-
- NantCell, Inc. QUILT‐3.040: ETBX‐011 (Ad5 [E1‐, E2b‐]‐CEA(6D)) vaccine in combination with ALT‐803 (super‐agonist IL‐15) in subjects having CEA‐expressing cancer. clinicaltrials.gov.
NCT03197584 {unpublished data only}
-
- NantKwest, Inc. QUILT‐3.051: NANT ovarian cancer vaccine: combination immunotherapy in subjects with epithelial ovarian cancer who have progressed on or after standard‐of‐care (SOC) therapy. clinicaltrials.gov.
NCT03206047 {unpublished data only}
-
- National Cancer Institute (NCI). Atezolizumab, Guadecitabine, and CDX‐1401 vaccine in treating patients with recurrent ovarian, fallopian tube, or primary peritoneal cancer. clinicaltrials.gov.
NCT03300843 {unpublished data only}
-
- National Cancer Institute (NCI). Ability of a dendritic cell vaccine to immunize melanoma or epithelial cancer patients against defined mutated neoantigens expressed by the autologous cancer. clinicaltrials.gov.
Additional references
Agarwal 2006
-
- Agarwal R, Linch M, Kaye SB. Novel therapeutic agents in ovarian cancer. European Journal of Surgical Oncology 2006;32(8):875‐86. - PubMed
Antonia 2006
-
- Antonia SJ, Mirza N, Fricke I, Chiappori A, Thompson P, Williams N, et al. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clinical Cancer Research 2006;12(3):878‐87. - PubMed
Brighton Collaboration 2009
-
- Brighton Collaboration. www.brightoncollaboration.org.
Britten 2008
-
- Britten CM, Gouttefangeas C, Welters MJ, Pawelec G, Koch S, Ottensmeier C, et al. The CIMT‐monitoring panel: a two‐step approach to harmonize the enumeration of antigen‐specific CD8+ T lymphocytes by structural and functional assays. Cancer Immunology, Immunotherapy 2008;57(3):289‐302. - PMC - PubMed
CTCAE 2009
-
- Common Terminology Criteria for Adverse Events v4.03. National Institutes of Health, US Department of Health and Human Services, 2009, publication # 09‐7473.
Deeks 2003
-
- Deeks JJ, Dinnes J, D'Amico R, Sowden AJ, Sakarovitch C, Song F, et al. International Stroke Trial Collaborative Group, European Carotid Surgery Trial Collaborative Group. Evaluating non‐randomised intervention studies. Health Technology Assessment 2003;7(27):1‐173. - PubMed
Dong 2006
-
- Dong HP, Elstrand MB, Holth A, Silins I, Berner A, Trope CG, et al. NK‐ and B‐cell infiltration correlates with worse outcome in metastatic ovarian carcinoma. American Journal of Clinical Pathology 2006;125(3):451‐8. - PubMed
Drerup 2015
Garnett 2008
Gooden 2011
Guyatt 2008
Hardwick 2016
-
- Hardwick N, Frankel PH, Cristea M. New approaches for immune directed treatment for ovarian cancer. Current Treatment Options in Oncology 2016;17(3):14. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Laheru 2008
-
- Laheru D, Lutz E, Burke J, Biedrzycki B, Solt S, Onners B. Allogeneic granulocyte macrophage colony‐stimulating factor‐secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clinical Cancer Research 2008;14(5):1455–63. - PMC - PubMed
Leffers 2009
-
- Leffers N, Daemen CAHH, Zee AGJ, Nijman HW. Multimodality treatment warranted for ovarian cancer: immunotherapy, a prerequisite to improve prognosis for this vicious disease. Immunotherapy 2009;1(2):163‐5. - PubMed
Odunsi 2017
Prat 2015
Raspollini 2005
-
- Raspollini MR, Castiglione F, Rossi Degl'innocenti D, Amunni G, Villanucci A, Garbini F, et al. Tumour‐infiltrating gamma/delta T‐lymphocytes are correlated with a brief disease‐free interval in advanced ovarian serous carcinoma. Annals of Oncology 2005;16(4):590‐6. - PubMed
Rustin 2004
-
- Rustin GJ, Quinn M, Thigpen T, du Bois A, Pujade‐Lauraine E, Jakobsen A, et al. Re: new guidelines to evaluate the response to treatment in solid tumors (ovarian cancer). Journal of the National Cancer Institute 2004;96(6):487‐8. - PubMed
Sato 2005
-
- Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor‐infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favourable prognosis in ovarian cancer. Proceedings of the National Academy of Sciences of the United States of America 2005;102(51):18538‐43. - PMC - PubMed
Therasse 2000
-
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. Journal of the National Cancer Institute 2000;92(3):205‐16. - PubMed
Thigpen 2000
-
- Thigpen JT. Chemotherapy for advanced ovarian cancer: overview of randomized trials. Seminars in Oncology 2000;27(3 Suppl 7):11‐6. - PubMed
Torre 2012
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. A Cancer Journal for Clinicians 2015;65(2):87‐108. - PubMed
Trotti 2003
-
- Trotti A, Colevas A, Setser V, Rusch D, Jaques V, Budach C, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Seminars in Radiation Oncology 2003;13(3):176‐81A. - PubMed
WHO 1979
-
- World Health Organization. Handbook for Reporting Results of Cancer Treatment. Geneva: World Health Organization, 1979.
Zhang 2003
-
- Zhang L, Conejo‐Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. New England Journal of Medicine 2003;348(3):203‐13. - PubMed
References to other published versions of this review
Leffers 2010
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

