Biologically Inspired Total Synthesis of Ulbactin F, an Iron-Binding Natural Product
- PMID: 30199265
- PMCID: PMC6456333
- DOI: 10.1021/acs.orglett.8b02599
Biologically Inspired Total Synthesis of Ulbactin F, an Iron-Binding Natural Product
Abstract
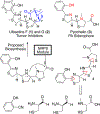
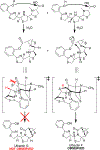
Natural products from environmental microbiomes provide exquisite templates for elucidating biological activity in the search for new drugs. A recently discovered marine Brevibacillus sp. metabolite, ulbactin F, was found to inhibit tumor cell migration and invasion at IC50 < 3 μM. Herein, we disclose the first total synthesis of ulbactin F and epi-ulbactin F, which was modeled after the biosynthetic pathway. The scaffold bears structural similarity to siderophores of human pathogens but contains a novel tricyclic ring system derived from cysteine. We have found that ulbactin F forms low-affinity metal complexes, with a preference for Fe3+ and Cu2+, which may hint both at its environmental role and its antimetastatic mechanism of action.
Figures

Similar articles
-
Ulbactins F and G, Polycyclic Thiazoline Derivatives with Tumor Cell Migration Inhibitory Activity from Brevibacillus sp.Org Lett. 2016 Apr 1;18(7):1658-61. doi: 10.1021/acs.orglett.6b00531. Epub 2016 Mar 21. Org Lett. 2016. PMID: 26998643
-
Stereoselective total synthesis and structural elucidation of (-)-indoxamycins A-F.Chemistry. 2014 Nov 10;20(46):15053-60. doi: 10.1002/chem.201403986. Epub 2014 Sep 26. Chemistry. 2014. PMID: 25262815
-
Our recent progress on the intramolecular Diels-Alder reaction approach in natural products synthesis: synthetic studies of the octahydronaphthalene substructure of versipelostatins and the A/B/C-tricyclic substructure of GKK1032s.Chem Rec. 2014 Aug;14(4):623-40. doi: 10.1002/tcr.201402008. Epub 2014 Jul 22. Chem Rec. 2014. PMID: 25049071
-
Natural Product-Based Antibiotics: Synthesis and SAR-Studies.Curr Pharm Des. 2016;22(12):1730-55. doi: 10.2174/1381612822666160115130633. Curr Pharm Des. 2016. PMID: 26769331 Review.
-
Total synthesis of marine natural products driven by novel structure, potent biological activity, and/or synthetic methodology.Ernst Schering Res Found Workshop. 2000;(32):103-48. doi: 10.1007/978-3-662-04042-3_4. Ernst Schering Res Found Workshop. 2000. PMID: 11077607 Review. No abstract available.
Cited by
-
Pyochelin Biosynthetic Metabolites Bind Iron and Promote Growth in Pseudomonads Demonstrating Siderophore-like Activity.ACS Infect Dis. 2021 Mar 12;7(3):544-551. doi: 10.1021/acsinfecdis.0c00897. Epub 2021 Feb 12. ACS Infect Dis. 2021. PMID: 33577297 Free PMC article.
-
The diversity and utility of arylthiazoline and aryloxazoline siderophores: challenges of total synthesis.RSC Adv. 2022 Sep 7;12(39):25284-25322. doi: 10.1039/d2ra03841b. eCollection 2022 Sep 5. RSC Adv. 2022. PMID: 36199325 Free PMC article. Review.
-
Unraveling Plant Natural Chemical Diversity for Drug Discovery Purposes.Front Pharmacol. 2020 Apr 7;11:397. doi: 10.3389/fphar.2020.00397. eCollection 2020. Front Pharmacol. 2020. PMID: 32317969 Free PMC article. Review.
-
Promiscuous Pseudomonas: Uptake of Non-Endogenous Ligands for Iron Acquisition.Tetrahedron Lett. 2021 Jul 6;75:153204. doi: 10.1016/j.tetlet.2021.153204. Epub 2021 May 24. Tetrahedron Lett. 2021. PMID: 34248214 Free PMC article.
-
Broad Spectrum Antibiotic Xanthocillin X Effectively Kills Acinetobacter baumannii via Dysregulation of Heme Biosynthesis.ACS Cent Sci. 2021 Mar 24;7(3):488-498. doi: 10.1021/acscentsci.0c01621. Epub 2021 Jan 20. ACS Cent Sci. 2021. PMID: 33791430 Free PMC article.
References
-
- Molinski TF; Dalisay DS; Lievens SL; Saludes JP Nat. Rev. Drug Discovery 2009, 8 (1), 69–85. - PubMed
-
- Igarashi Y; Asano D; Sawamura M; In Y; Ishida T; Imoto M Org. Lett. 2016, 18 (7), 1658–1661. - PubMed
-
- Eleftherianos I; Dowling A; Wilkinson P; Parkhill J; et al. Proc. Natl. Acad. Sci. U. S. A. 2009, 106 (6), 6–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

