Biologically Inspired Total Synthesis of Ulbactin F, an Iron-Binding Natural Product
- PMID: 30199265
- PMCID: PMC6456333
- DOI: 10.1021/acs.orglett.8b02599
Biologically Inspired Total Synthesis of Ulbactin F, an Iron-Binding Natural Product
Abstract
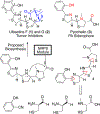
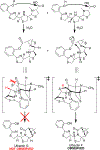
Natural products from environmental microbiomes provide exquisite templates for elucidating biological activity in the search for new drugs. A recently discovered marine Brevibacillus sp. metabolite, ulbactin F, was found to inhibit tumor cell migration and invasion at IC50 < 3 μM. Herein, we disclose the first total synthesis of ulbactin F and epi-ulbactin F, which was modeled after the biosynthetic pathway. The scaffold bears structural similarity to siderophores of human pathogens but contains a novel tricyclic ring system derived from cysteine. We have found that ulbactin F forms low-affinity metal complexes, with a preference for Fe3+ and Cu2+, which may hint both at its environmental role and its antimetastatic mechanism of action.
Figures

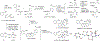
References
-
- Molinski TF; Dalisay DS; Lievens SL; Saludes JP Nat. Rev. Drug Discovery 2009, 8 (1), 69–85. - PubMed
-
- Igarashi Y; Asano D; Sawamura M; In Y; Ishida T; Imoto M Org. Lett. 2016, 18 (7), 1658–1661. - PubMed
-
- Eleftherianos I; Dowling A; Wilkinson P; Parkhill J; et al. Proc. Natl. Acad. Sci. U. S. A. 2009, 106 (6), 6–12.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

