Long-Term Safety and Efficacy of Minimally Invasive Lumbar Decompression Procedure for the Treatment of Lumbar Spinal Stenosis With Neurogenic Claudication: 2-Year Results of MiDAS ENCORE
- PMID: 30199512
- PMCID: PMC6319572
- DOI: 10.1097/AAP.0000000000000868
Long-Term Safety and Efficacy of Minimally Invasive Lumbar Decompression Procedure for the Treatment of Lumbar Spinal Stenosis With Neurogenic Claudication: 2-Year Results of MiDAS ENCORE
Abstract
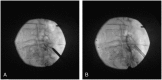
Background and objectives: This study evaluated the long-term durability of the minimally invasive lumbar decompression (MILD) procedure in terms of functional improvement and pain reduction for patients with lumbar spinal stenosis and neurogenic claudication due to hypertrophic ligamentum flavum. This is a report of 2-year follow-up for MILD study patients.
Methods: This prospective, multicenter, randomized controlled clinical study compared outcomes for 143 patients treated with MILD versus 131 treated with epidural steroid injections. Follow-up occurred at 6 months and at 1 year for the randomized phase and at 2 years for MILD subjects only. Oswestry Disability Index, Numeric Pain Rating Scale, and Zurich Claudication Questionnaire were used to evaluate function and pain. Safety was evaluated by assessing incidence of device-/procedure-related adverse events.
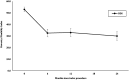
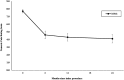
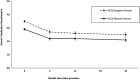
Results: All outcome measures demonstrated clinically meaningful and statistically significant improvement from baseline through 6-month, 1-year, and 2-year follow-ups. At 2 years, Oswestry Disability Index improved by 22.7 points, Numeric Pain Rating Scale improved by 3.6 points, and Zurich Claudication Questionnaire symptom severity and physical function domains improved by 1.0 and 0.8 points, respectively. There were no serious device-/procedure-related adverse events, and 1.3% experienced a device-/procedure-related adverse event.
Conclusions: MILD showed excellent long-term durability, and there was no evidence of spinal instability through 2-year follow-up. Reoperation and spinal fracture rates are lower, and safety is higher for MILD versus other lumbar spine interventions, including interspinous spacers, surgical decompression, and spinal fusion. Given the minimally invasive nature of this procedure, its robust success rate, and durability of outcomes, MILD is an excellent choice for first-line therapy for select patients with central spinal stenosis suffering from neurogenic claudication symptoms with hypertrophic ligamentum flavum.
Clinical trial registration: This study was registered at ClinicalTrials.gov, identifier NCT02093520.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
MILD® Is an Effective Treatment for Lumbar Spinal Stenosis with Neurogenic Claudication: MiDAS ENCORE Randomized Controlled Trial.Pain Physician. 2016 May;19(4):229-42. Pain Physician. 2016. PMID: 27228511 Clinical Trial.
-
MiDAS ENCORE: Randomized Controlled Clinical Trial Report of 6-Month Results.Pain Physician. 2016 Feb;19(2):25-38. Pain Physician. 2016. PMID: 26815247 Clinical Trial.
-
MiDAS I (mild Decompression Alternative to Open Surgery): a preliminary report of a prospective, multi-center clinical study.Pain Physician. 2010 Jul-Aug;13(4):369-78. Pain Physician. 2010. PMID: 20648206 Clinical Trial.
-
Minimally invasive lumbar decompression: a review of indications, techniques, efficacy and safety.Pain Manag. 2020 Sep;10(5):331-348. doi: 10.2217/pmt-2020-0037. Epub 2020 Jul 1. Pain Manag. 2020. PMID: 32609052 Review.
-
The Efficacy of the Minimally Invasive Lumbar Decompression (MILD®) Procedure: A PRISMA-compliant Systemic Review and Meta-analysis.Pain Physician. 2025 Mar;28(2):71-81. Pain Physician. 2025. PMID: 40168556
Cited by
-
Artificial Intelligence Algorithm-Based Lumbar and Spinal MRI for Evaluation of Efficacy of Chinkuei Shin Chewan Decoction on Lumbar Spinal Stenosis.Contrast Media Mol Imaging. 2021 Dec 29;2021:2700452. doi: 10.1155/2021/2700452. eCollection 2021. Contrast Media Mol Imaging. 2021. PMID: 35035312 Free PMC article. Clinical Trial.
-
The use of minimally invasive interspinous process devices for the treatment of lumbar canal stenosis: a narrative literature review.J Spine Surg. 2021 Sep;7(3):394-412. doi: 10.21037/jss-21-57. J Spine Surg. 2021. PMID: 34734144 Free PMC article. Review.
-
Pacific Spine and Pain Society (PSPS) Evidence Review of Surgical Treatments for Lumbar Degenerative Spinal Disease: A Narrative Review.Pain Ther. 2024 Jun;13(3):349-390. doi: 10.1007/s40122-024-00588-4. Epub 2024 Mar 23. Pain Ther. 2024. PMID: 38520658 Free PMC article. Review.
-
Minimally Invasive Lumbar Decompression and Interspinous Process Device for the Management of Symptomatic Lumbar Spinal Stenosis: a Literature Review.Curr Pain Headache Rep. 2020 Feb 18;24(4):13. doi: 10.1007/s11916-020-0845-2. Curr Pain Headache Rep. 2020. PMID: 32072362
-
Lumbar Spinal Stenosis and Minimally Invasive Lumbar Decompression: A Narrative Review.J Pain Res. 2023 Nov 6;16:3707-3724. doi: 10.2147/JPR.S428112. eCollection 2023. J Pain Res. 2023. PMID: 37954472 Free PMC article. Review.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
