The Future of Influenza Vaccines: A Historical and Clinical Perspective
- PMID: 30200179
- PMCID: PMC6160951
- DOI: 10.3390/vaccines6030058
The Future of Influenza Vaccines: A Historical and Clinical Perspective
Abstract
For centuries, the development of vaccines to prevent infectious disease was an empirical process. From smallpox variolation in Song dynasty China, through the polysaccharide capsule vaccines developed in the 1970s, vaccines were made either from the pathogen itself, treated in some way to render it attenuated or non-infectious, or from a closely related non-pathogenic strain. In recent decades, new scientific knowledge and technologies have enabled rational vaccine design in a way that was unimaginable before. However, vaccines optimal against some infectious diseases, influenza among them, have remained elusive. This review will highlight the challenges that influenza viruses pose for rational vaccine design. In particular, it will consider the clinically beneficial endpoints, beyond complete sterilizing immunity, that have been achieved with vaccines against other infectious diseases, as well as the barriers to achieving similar success against influenza.
Keywords: effectiveness; efficacy; influenza; morbidity; mortality; transmission; vaccine.
Conflict of interest statement
The author declares no conflict of interest.
Figures

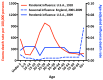
References
-
- Grohskopf L.A., Sokolow L.Z., Broder K.R., Walter E.B., Bresee J.S., Fry A.M., Jernigan D.B. Prevention and control of seasonal influenza with vaccines: Recommendations of the advisory committee on immunization practices—United States, 2017–2018 influenza season. MMWR Recomm. Rep. 2017;66:1–20. doi: 10.15585/mmwr.rr6602a1. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

