Antiproliferative Activity of Non-Calcemic Vitamin D Analogs on Human Melanoma Lines in Relation to VDR and PDIA3 Receptors
- PMID: 30200275
- PMCID: PMC6163194
- DOI: 10.3390/ijms19092583
Antiproliferative Activity of Non-Calcemic Vitamin D Analogs on Human Melanoma Lines in Relation to VDR and PDIA3 Receptors
Abstract
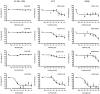
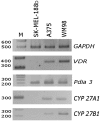
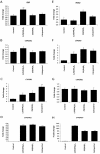
Vitamin D is a precursor for secosteroidal hormones, which demonstrate pleiotropic biological activities, including the regulation of growth and the differentiation of normal and malignant cells. Our previous studies have indicated that the inhibition of melanoma proliferation by a short side-chain, low calcemic analog of vitamin D-21(OH)pD is not fully dependent on the expression of vitamin D receptor (VDR). We have examined the effects of classic vitamin D metabolites, 1,25(OH)₂D₃ and 25(OH)D₃, and two low calcemic vitamin D analogs, (21(OH)pD and calcipotriol), on proliferation, mRNA expression and vitamin D receptor (VDR) translocation in three human melanoma cell lines: WM98, A375 and SK-MEL-188b (subline b of SK-MEL-188, which lost responsiveness to 1,25(OH)₂D₃ and became VDR-/-CYP27B1-/-). All tested compounds efficiently inhibited the proliferation of WM98 and A375 melanoma cells except SK-MEL-188b, in which only the short side-chain vitamin D analog-21(OH)pD was effective. Overall, 21(OH)pD was the most potent compound in all three melanoma cell lines in the study. The lack of responsiveness of SK-MEL-188b to 1,25(OH)₂D₃, 25(OH)D₃ and calcipotriol is explained by a lack of characteristic transcripts for the VDR, its splicing variants as well as for vitamin D-activating enzyme CYP27B1. On the other hand, the expression of VDR and its splicing variants and other vitamin D related genes (RXR, PDIA3, CYP3A4, CYP2R1, CYP27B1, CYP24A1 and CYP11A1) was detected in WM98 and A375 melanomas with the transcript levels being modulated by vitamin D analogs. The expression of VDR isoforms in WM98 cells was stimulated strongly by calcipotriol. The antiproliferative activities of 21(OH)pD appear not to require VDR translocation to the nucleus, which explains the high efficacy of this noncalcemic pregnacalciferol analog in SK-MEL-188b melanoma, that is, VDR-/-. Therefore, we propose that 21(OH)pD is a good candidate for melanoma therapy, although the mechanism of its action remains to be defined.
Keywords: 1,25(OH)2D3; 21(OH)pD; VDR translocation; anti-melanoma activity; calcipotriol; human melanoma cell lines; melanoma; vitamin D; vitamin D analogs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
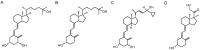


Similar articles
-
In vitro comparison of the vitamin D endocrine system in 1,25(OH)2D3-responsive and -resistant melanoma cells.Cancer Biol Ther. 2007 Jan;6(1):48-55. doi: 10.4161/cbt.6.1.3493. Cancer Biol Ther. 2007. PMID: 17172823
-
Anticancer Activity of Vitamin D, Lumisterol and Selected Derivatives against Human Malignant Melanoma Cell Lines.Int J Mol Sci. 2024 Oct 10;25(20):10914. doi: 10.3390/ijms252010914. Int J Mol Sci. 2024. PMID: 39456696 Free PMC article.
-
Antiproliferative activity of side-chain truncated vitamin D analogs (PRI-1203 and PRI-1204) against human malignant melanoma cell lines.Eur J Pharmacol. 2020 Aug 15;881:173170. doi: 10.1016/j.ejphar.2020.173170. Epub 2020 May 20. Eur J Pharmacol. 2020. PMID: 32445704
-
Vitamin D and systemic cancer: is this relevant to malignant melanoma?Br J Dermatol. 2002 Aug;147(2):197-213. doi: 10.1046/j.1365-2133.2002.04960.x. Br J Dermatol. 2002. PMID: 12174089 Review.
-
Metabolism and Action of 25-Hydroxy-19-nor-Vitamin D₃ in Human Prostate Cells.Vitam Horm. 2016;100:357-77. doi: 10.1016/bs.vh.2015.10.009. Epub 2015 Dec 8. Vitam Horm. 2016. PMID: 26827959 Review.
Cited by
-
The Variability of Vitamin D Concentrations in Short Children with Short Stature from Central Poland-The Effects of Insolation, Supplementation, and COVID-19 Pandemic Isolation.Nutrients. 2023 Aug 18;15(16):3629. doi: 10.3390/nu15163629. Nutrients. 2023. PMID: 37630820 Free PMC article.
-
The Role of Classical and Novel Forms of Vitamin D in the Pathogenesis and Progression of Nonmelanoma Skin Cancers.Adv Exp Med Biol. 2020;1268:257-283. doi: 10.1007/978-3-030-46227-7_13. Adv Exp Med Biol. 2020. PMID: 32918223 Free PMC article. Review.
-
Fibroblast Growth Factor Receptor Inhibitors Decrease Proliferation of Melanoma Cell Lines and Their Activity Is Modulated by Vitamin D.Int J Mol Sci. 2024 Feb 21;25(5):2505. doi: 10.3390/ijms25052505. Int J Mol Sci. 2024. PMID: 38473753 Free PMC article.
-
Vitamin D Modulates the Response of Patient-Derived Metastatic Melanoma Cells to Anticancer Drugs.Int J Mol Sci. 2023 Apr 28;24(9):8037. doi: 10.3390/ijms24098037. Int J Mol Sci. 2023. PMID: 37175742 Free PMC article.
-
Relevance of Vitamin D in Melanoma Development, Progression and Therapy.Anticancer Res. 2020 Jan;40(1):473-489. doi: 10.21873/anticanres.13976. Anticancer Res. 2020. PMID: 31892603 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

