Injured Achilles Tendons Treated with Adipose-Derived Stem Cells Transplantation and GDF-5
- PMID: 30200326
- PMCID: PMC6162699
- DOI: 10.3390/cells7090127
Injured Achilles Tendons Treated with Adipose-Derived Stem Cells Transplantation and GDF-5
Abstract
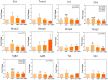
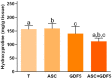
Tendon injuries represent a clinical challenge in regenerative medicine because their natural repair process is complex and inefficient. The high incidence of tendon injuries is frequently associated with sports practice, aging, tendinopathies, hypertension, diabetes mellitus, and the use of corticosteroids. The growing interest of scientists in using adipose-derived mesenchymal stem cells (ADMSC) in repair processes seems to be mostly due to their paracrine and immunomodulatory effects in stimulating specific cellular events. ADMSC activity can be influenced by GDF-5, which has been successfully used to drive tenogenic differentiation of ADMSC in vitro. Thus, we hypothesized that the application of ADMSC in isolation or in association with GDF-5 could improve Achilles tendon repair through the regulation of important remodeling genes expression. Lewis rats had tendons distributed in four groups: Transected (T), transected and treated with ADMSC (ASC) or GDF-5 (GDF5), or with both (ASC+GDF5). In the characterization of cells before application, ADMSC expressed the positive surface markers, CD90 (90%) and CD105 (95%), and the negative marker, CD45 (7%). ADMSC were also differentiated in chondrocytes, osteoblast, and adipocytes. On the 14th day after the tendon injury, GFP-ADMSC were observed in the transected region of tendons in the ASC and ASC+GDF5 groups, and exhibited and/or stimulated a similar genes expression profile when compared to the in vitro assay. ADMSC up-regulated Lox, Dcn, and Tgfb1 genes expression in comparison to T and ASC+GDF5 groups, which contributed to a lower proteoglycans arrangement, and to a higher collagen fiber organization and tendon biomechanics in the ASC group. The application of ADMSC in association with GDF-5 down-regulated Dcn, Gdf5, Lox, Tgfb1, Mmp2, and Timp2 genes expression, which contributed to a lower hydroxyproline concentration, lower collagen fiber organization, and to an improvement of the rats' gait 24 h after the injury. In conclusion, although the literature describes the benefic effect of GDF-5 for the tendon healing process, our results show that its application, isolated or associated with ADMSC, cannot improve the repair process of partial transected tendons, indicating the higher effectiveness of the application of ADMSC in injured Achilles tendons. Our results show that the application of ADMSC in injured Achilles tendons was more effective in relation to its association with GDF-5.
Keywords: biomechanics; collagen; extracellular matrix; gait; gene expression; repair.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

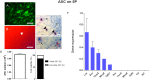
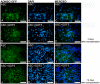
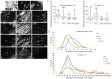
 ); observe the crimp (B) by positioning the largest axis of the tendon parallel to one of the polarizers: The same region observed in (A). (C) Panoramic image of the transected tendon for identification of the transection region (TR) and the proximal and distal transition region (T1). Groups T (D–F), ASC (G–I), GDF5 (J–L), and ASC+GDF5 (M–O). Observe the complete disorganization of collagen fibers in TR. The TR from different groups (D,G,J,M): Observe freshly formed collagen fibrils and an overlapping (↘) of this region with the thicker fibers present in T1. T1 (E,H,K,N): Collagen fibers with a greater organization in relation to TR, however, with fragmentation presence (◢) mainly in groups T (E), GDF5 (K), and ASC+GDF5 (N). Crimp (F,I,L,O) from the collagen fibers observed on T1: Observe similar undulation patterns of the collagen fibers between the groups, represented by light and dark regions. The largest axis of the tendon was positioned at 45° in relation to the crossed polarizers as parallel to one of the polarizers (B,F,I,L,O). (P) TR birefringence measurements in T1: Same letter represents significant differences between groups (p ≤ 0.05). (Q) Histogram of the frequency and birefringence values showing differences in the distribution of values in the different groups. Bars = 100 μm and 200 μm (a).
); observe the crimp (B) by positioning the largest axis of the tendon parallel to one of the polarizers: The same region observed in (A). (C) Panoramic image of the transected tendon for identification of the transection region (TR) and the proximal and distal transition region (T1). Groups T (D–F), ASC (G–I), GDF5 (J–L), and ASC+GDF5 (M–O). Observe the complete disorganization of collagen fibers in TR. The TR from different groups (D,G,J,M): Observe freshly formed collagen fibrils and an overlapping (↘) of this region with the thicker fibers present in T1. T1 (E,H,K,N): Collagen fibers with a greater organization in relation to TR, however, with fragmentation presence (◢) mainly in groups T (E), GDF5 (K), and ASC+GDF5 (N). Crimp (F,I,L,O) from the collagen fibers observed on T1: Observe similar undulation patterns of the collagen fibers between the groups, represented by light and dark regions. The largest axis of the tendon was positioned at 45° in relation to the crossed polarizers as parallel to one of the polarizers (B,F,I,L,O). (P) TR birefringence measurements in T1: Same letter represents significant differences between groups (p ≤ 0.05). (Q) Histogram of the frequency and birefringence values showing differences in the distribution of values in the different groups. Bars = 100 μm and 200 μm (a).

Similar articles
-
Transected Tendon Treated with a New Fibrin Sealant Alone or Associated with Adipose-Derived Stem Cells.Cells. 2019 Jan 16;8(1):56. doi: 10.3390/cells8010056. Cells. 2019. PMID: 30654437 Free PMC article.
-
Low-level laser and adipose-derived stem cells altered remodelling genes expression and improved collagen reorganization during tendon repair.Cell Prolif. 2019 May;52(3):e12580. doi: 10.1111/cpr.12580. Epub 2019 Feb 7. Cell Prolif. 2019. PMID: 30734394 Free PMC article.
-
Microcurrent and adipose-derived stem cells modulate genes expression involved in the structural recovery of transected tendon of rats.FASEB J. 2020 Aug;34(8):10011-10026. doi: 10.1096/fj.201902942RR. Epub 2020 Jun 19. FASEB J. 2020. PMID: 32558993
-
Directed Differentiation and Paracrine Mechanisms of Mesenchymal Stem Cells: Potential Implications for Tendon Repair and Regeneration.Curr Stem Cell Res Ther. 2017;12(6):447-454. doi: 10.2174/1574888X12666170502102423. Curr Stem Cell Res Ther. 2017. PMID: 28464787 Review.
-
Growth and Development Symposium: Stem cell therapy in equine tendon injury.J Anim Sci. 2013 Jan;91(1):59-65. doi: 10.2527/jas.2012-5736. Epub 2012 Oct 16. J Anim Sci. 2013. PMID: 23100589 Review.
Cited by
-
Tendon-Specific Activation of Tenogenic Transcription Factors Enables Keeping Tenocytes' Identity In Vitro.Int J Mol Sci. 2022 Nov 15;23(22):14078. doi: 10.3390/ijms232214078. Int J Mol Sci. 2022. PMID: 36430562 Free PMC article.
-
Tendon stem/progenitor cell ageing: Modulation and rejuvenation.World J Stem Cells. 2019 Sep 26;11(9):677-692. doi: 10.4252/wjsc.v11.i9.677. World J Stem Cells. 2019. PMID: 31616543 Free PMC article. Review.
-
Injectable self-assembled GDF5-containing dipeptide hydrogels for enhanced tendon repair.Mater Today Bio. 2024 Apr 3;26:101046. doi: 10.1016/j.mtbio.2024.101046. eCollection 2024 Jun. Mater Today Bio. 2024. PMID: 38600922 Free PMC article.
-
The roles and therapeutic potentialof mesenchymal stem/stromal cells and their extracellular vesicles in tendinopathies.Front Bioeng Biotechnol. 2023 Jan 19;11:1040762. doi: 10.3389/fbioe.2023.1040762. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 36741745 Free PMC article. Review.
-
A Tendon-Specific Double Reporter Transgenic Mouse Enables Tracking Cell Lineage and Functions Alteration In Vitro and In Vivo.Int J Mol Sci. 2021 Oct 17;22(20):11189. doi: 10.3390/ijms222011189. Int J Mol Sci. 2021. PMID: 34681849 Free PMC article.
References
-
- Vidal B.C., Mello M.L. Proteoglycan arrangement in tendon collagen bundles. Cell Mol. Biol. 1984;30:195–204. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

