Recombinant Escherichia coli BL21-pET28a- egfp Cultivated with Nanomaterials in a Modified Microchannel for Biofilm Formation
- PMID: 30200345
- PMCID: PMC6163294
- DOI: 10.3390/ijms19092590
Recombinant Escherichia coli BL21-pET28a- egfp Cultivated with Nanomaterials in a Modified Microchannel for Biofilm Formation
Abstract
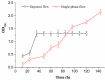
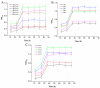
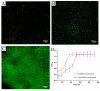
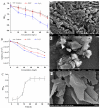
The application of whole cells as catalytic biofilms in microchannels has attracted increasing scientific interest. However, the excessive biomass formation and structure of biofilms in a reactor limits their use. A microchannel reactor with surface modification was used to colonize recombinant Escherichia coil BL21-pET28a-egfp rapidly and accelerated growth of biofilms in the microchannel. The segmented flow system of 'air/culture medium containing nanomaterials' was firstly used to modulate the biofilms formation of recombinant E. coil; the inhibitory effects of nanomaterials on biofilm formation were investigated. The results indicated that the segmental flow mode has a significant impact on the structure and development of biofilms. Using the channels modified by silane reagent, the culture time of biofilms (30 h) was reduced by 6 h compared to unmodified channels. With the addition of graphene sheets (10 mg/L) in Luria-Bertani (LB) medium, the graphene sheets possessed a minimum inhibition rate of 3.23% against recombinant E. coil. The biofilms cultivated by the LB medium with added graphene sheets were stably formed in 20 h; the formation time was 33.33% shorter than that by LB medium without graphene. The developed method provides an efficient and simple approach for rapid preparation of catalytic biofilms in microchannel reactors.
Keywords: biofilm; microreactor; nanomaterials; recombinant Escherichia coil; surface modification.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Enhanced high copy number plasmid maintenance and heterologous protein production in an Escherichia coli biofilm.Biotechnol Bioeng. 2007 Jun 15;97(3):439-46. doi: 10.1002/bit.21240. Biotechnol Bioeng. 2007. PMID: 17058286
-
Moving and unsinkable graphene sheets immobilized enzyme for microfluidic biocatalysis.Sci Rep. 2017 Jun 27;7(1):4309. doi: 10.1038/s41598-017-04216-4. Sci Rep. 2017. PMID: 28655888 Free PMC article.
-
Effects of antibiotic concentration and nutrient medium composition on Escherichia coli biofilm formation and green fluorescent protein expression.FEMS Microbiol Lett. 2017 Mar 1;364(5). doi: 10.1093/femsle/fnx042. FEMS Microbiol Lett. 2017. PMID: 28364732
-
"It's a gut feeling" - Escherichia coli biofilm formation in the gastrointestinal tract environment.Crit Rev Microbiol. 2018 Feb;44(1):1-30. doi: 10.1080/1040841X.2017.1303660. Epub 2017 May 9. Crit Rev Microbiol. 2018. PMID: 28485690 Review.
-
Microbial biofilms: a concept for industrial catalysis?Trends Biotechnol. 2009 Nov;27(11):636-43. doi: 10.1016/j.tibtech.2009.08.001. Epub 2009 Sep 23. Trends Biotechnol. 2009. PMID: 19783314 Review.
Cited by
-
The Transcription Factor CsgD Contributes to Engineered Escherichia coli Resistance by Regulating Biofilm Formation and Stress Responses.Int J Mol Sci. 2023 Sep 5;24(18):13681. doi: 10.3390/ijms241813681. Int J Mol Sci. 2023. PMID: 37761984 Free PMC article.
References
-
- Maksimova Y.G. Microbial biofilms in biotechnological processes. Appl. Biochem. Microbiol. 2014;50:750–760. doi: 10.1134/S0003683814080043. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

