Tracing the Thermal History of Seafood Products through Lysophospholipid Analysis by Hydrophilic Interaction Liquid Chromatography⁻Electrospray Ionization Fourier Transform Mass Spectrometry
- PMID: 30200346
- PMCID: PMC6225239
- DOI: 10.3390/molecules23092212
Tracing the Thermal History of Seafood Products through Lysophospholipid Analysis by Hydrophilic Interaction Liquid Chromatography⁻Electrospray Ionization Fourier Transform Mass Spectrometry
Abstract
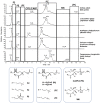
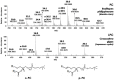
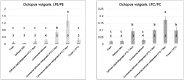
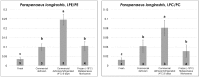
Low temperature treatments commonly applied to seafood products have been shown to influence their phospholipid (PL) profile through enzymatic hydrolysis. In the present study, the generation of lysophospholipids (LPL) resulting from this process was systematically investigated for selected, commercially relevant seafood products, namely oysters, clams, octopuses, and shrimps. These products were subjected to thermal treatments like refrigeration or freezing after being purchased as fresh, defrozen, or frozen products depending on the case. The coupling between hydrophilic interaction liquid chromatography (HILIC) and electrospray ionization with high resolution/accuracy Fourier transform mass spectrometry (ESI-FTMS) was exploited to evaluate the PL profile of the cited products, especially the incidence of LPL related to the two main PL classes of seafood products-phosphatidylcholines (PC) and phosphatidylethanolamines (PE)-in the lipid extracts. The lyso forms of PE (LPE) were found to be generally more sensitive than those of PC (LPC) to thermal treatments, usually exhibiting a significant increase upon prolonged refrigeration at 4 °C in all types of investigated products except European flat oysters. Moreover, the distinction between fresh and frozen or defrozen products could be achieved in the case of octopuses and shrimps, respectively.
Keywords: high resolution mass spectrometry; hydrophilic interaction liquid chromatography; lysophospholipids; seafood products; thermal treatments.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses or interpretation of data, in the writing of the manuscript, and in the decision to publish the results.
Figures

References
-
- Ackman R.G. Nutritional composition of fats in seafoods. Prog. Food Nutr. Sci. 1989;13:161–289. - PubMed
-
- Daviglus M., Sheeshka J., Murkin E. Health benefits from eating fish. Comments Toxicol. 2002;8:345–374. doi: 10.1080/08865140215064. - DOI
-
- Grazyna Daczkowska-Kozon E., Pan B.S. Environmental Effects on Seafood Availability, Safety and Quality. 2nd ed. CRC Press; Boca Raton, FL, USA: 2011.
-
- Food and Agriculture Organization of the United Nations (FAO) The State of World Fisheries and Aquaculture. [(accessed on 5 June 2018)];2016 Available online: http://www.fao.org/3/a-i5555e.pdf.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

