Efficacy of an Oral Rehydration Solution Enriched with Lactobacillus reuteri DSM 17938 and Zinc in the Management of Acute Diarrhoea in Infants: A Randomized, Double-Blind, Placebo-Controlled Trial
- PMID: 30200394
- PMCID: PMC6165178
- DOI: 10.3390/nu10091189
Efficacy of an Oral Rehydration Solution Enriched with Lactobacillus reuteri DSM 17938 and Zinc in the Management of Acute Diarrhoea in Infants: A Randomized, Double-Blind, Placebo-Controlled Trial
Abstract
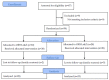
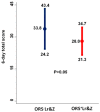
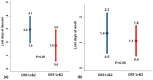
The efficacy of oral rehydration solution (ORS) enriched with Lactobacillus reuteri DSM 17938 and zinc in infants with acute gastroenteritis, is poorly defined. The aim of this double-blind, randomized, placebo-controlled study, was to assess the efficacy of an ORS enriched with Lactobacillus reuteri DSM 17938 and zinc (ORS⁺Lr&Z) in well-nourished, non-hospitalized infants with acute diarrhoea. Fifty one infants with acute diarrhoea were randomly assigned to receive either ORS⁺Lr&Z (28 infants, mean ± SD age 1.7 ± 0.7 years, 21 males), or standard ORS (ORS-Lr&Z; 23 infants, mean ± SD age 1.8 ± 0.7 years, 16 males). Stools volume and consistency were recorded pre- and posttreatment using the Amsterdam Infant Stool Scale and were compared between the two groups, as well as lost work/day care days, drug administration and need for hospitalization. Both groups showed reduction in the severity of diarrhoea on day two (p < 0.001) while, all outcomes showed a trend to be better in the ORS⁺Lr&Z group, without reaching statistical significance, probably due to the relatively small number of patients. No adverse effects were recorded. In conclusion, both ORS were effective in managing acute diarrhoea in well-nourished, non-hospitalized infants. ORS enriched with L. reuteri DSM 17938 and zinc was well tolerated with no adverse effects.
Keywords: Lactobacillus reuteri; acute gastroenteritis; children; oral rehydration solution; probiotics; zinc.
Conflict of interest statement
M.M., G.C., A.M., A.O. and A.P. received research grants from BioGaia, the manufacturer of the ORS enriched with
Figures


References
-
- World Health Organization, Dept. of Child and Adolescent Health and Development & UNICEF . Clinical Management of Acute Diarrhoea: WHO/UNICEF Joint Statement. World Health Organization; Geneva, Switzerland: 2004.
-
- Rosenfeldt V., Michaelsen K.F., Jakobsen M., Larsen C.N., Møller P.L., Pedersen P., Tvede M., Weyrehter H., Valerius N.H., Paerregaard A. Effect of probiotic Lactobacillus strains in young children hospitalized with acute diarrhea. Pediatr. Infect. Dis. J. 2002;21:411–416. doi: 10.1097/00006454-200205000-00012. - DOI - PubMed
-
- Rosenfeldt V., Michaelsen K.F., Jakobsen M., Larsen C.N., Møller P.L., Tvede M., Weyrehter H., Valerius N.H., Paerregaard A. Effect of probiotic Lactobacillus strains on acute diarrhea in a cohort of non-hospitalized children attending day-care centers. Pediatr. Infect. Dis. J. 2002;21:417–419. doi: 10.1097/00006454-200205000-00013. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

