Genetic Renal Diseases: The Emerging Role of Zebrafish Models
- PMID: 30200518
- PMCID: PMC6162634
- DOI: 10.3390/cells7090130
Genetic Renal Diseases: The Emerging Role of Zebrafish Models
Abstract
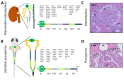
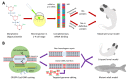
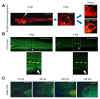
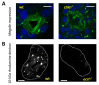
The structural and functional similarity of the larval zebrafish pronephros to the human nephron, together with the recent development of easier and more precise techniques to manipulate the zebrafish genome have motivated many researchers to model human renal diseases in the zebrafish. Over the last few years, great advances have been made, not only in the modeling techniques of genetic diseases in the zebrafish, but also in how to validate and exploit these models, crossing the bridge towards more informative explanations of disease pathophysiology and better designed therapeutic interventions in a cost-effective in vivo system. Here, we review the significant progress in these areas giving special attention to the renal phenotype evaluation techniques. We further discuss the future applications of such models, particularly their role in revealing new genetic diseases of the kidney and their potential use in personalized medicine.
Keywords: CRISPR; genetic renal diseases; morpholino; new therapies; pathophysiology; pronephros; zebrafish.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

