Influence of Dlutaraldehyde Cross-Linking Modes on the Recyclability of Immobilized Lipase B from Candida antarctica for Transesterification of Soy Bean Oil
- PMID: 30200521
- PMCID: PMC6225267
- DOI: 10.3390/molecules23092230
Influence of Dlutaraldehyde Cross-Linking Modes on the Recyclability of Immobilized Lipase B from Candida antarctica for Transesterification of Soy Bean Oil
Abstract
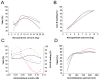
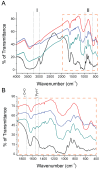
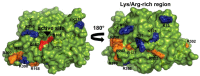
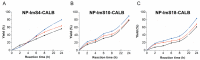
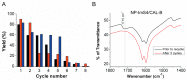
Lipase B from Candida antarctica (CAL-B) is largely employed as a biocatalyst for hydrolysis, esterification, and transesterification reactions. CAL-B is a good model enzyme to study factors affecting the enzymatic structure, activity and/or stability after an immobilization process. In this study, we analyzed the immobilization of CAL-B enzyme on different magnetic nanoparticles, synthesized by the coprecipitation method inside inverse micelles made of zwitterionic surfactants, with distinct carbon chain length: 4 (ImS4), 10 (ImS10) and 18 (ImS18) carbons. Magnetic nanoparticles ImS4 and ImS10 were shown to cross-link to CAL-B enzyme via a Michael-type addition, whereas particles with ImS18 were bond via pyridine formation after glutaraldehyde cross-coupling. Interestingly, the Michael-type cross-linking generated less stable immobilized CAL-B, revealing the influence of a cross-linking mode on the resulting biocatalyst behavior. Curiously, a direct correlation between nanoparticle agglomerate sizes and CAL-B enzyme reuse stability was observed. Moreover, free CAL-B enzyme was not able to catalyze transesterification due to the high methanol concentration; however, the immobilized CAL-B enzyme reached yields from 79.7 to 90% at the same conditions. In addition, the transesterification of lipids isolated from oleaginous yeasts achieved 89% yield, which confirmed the potential of immobilized CAL-B enzyme in microbial production of biodiesel.
Keywords: biodiesel synthesis; cross-linking types; enzyme immobilization; lipase; magnetic nanoparticles.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
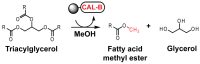

References
-
- Sirisha V.L., Jain A., Jain A. Enzyme immobilization: An overview on methods, support material, and applications of immobilized enzymes. Adv. Food Nutr. Res. 2016;79:179–211. - PubMed
-
- Netto C.G.C.M., Toma H.E., Andrade L.H. Superparamagnetic nanoparticles as versatile carriers and supporting materials for enzymes. J. Mol. Catal. B-Enzym. 2013;85–86:71–92. doi: 10.1016/j.molcatb.2012.08.010. - DOI
-
- Zdarta J., Meyer A.S., Jesionowski T., Pinelo M. A general overview of support materials for enzyme immobilization: Characteristics, properties, practical utility. Catalysts. 2018;8:92. doi: 10.3390/catal8020092. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

