Development of a Sensitive Enzyme-Linked Immunosorbent Assay and Rapid Gold Nanoparticle Immunochromatographic Strip for Detecting Citrinin in Monascus Fermented Food
- PMID: 30200526
- PMCID: PMC6162752
- DOI: 10.3390/toxins10090354
Development of a Sensitive Enzyme-Linked Immunosorbent Assay and Rapid Gold Nanoparticle Immunochromatographic Strip for Detecting Citrinin in Monascus Fermented Food
Abstract
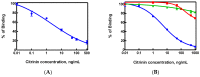
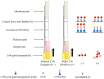
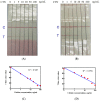
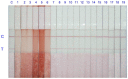
Antibodies against citrinin (CTN) were generated from rabbits, which were injected with CTN-keyhole limpet hemocyanin (KLH). This work involved the development of a sensitive competitive direct enzyme-linked immunosorbent assay (cdELISA) and a rapid gold nanoparticle immunochromatographic strip (immunostrip) method for analyzing CTN in Monascus-fermented food. CTN at a concentration of 5.0 ng/mL caused 50% inhibition (IC50) of CTN-horseradish peroxidase (CTN-HRP) binding to the antibodies in the cdELISA. The capable on-site detection of CTN was accomplished by a rapid antibody-gold nanoparticle immunostrip with a detection limit of 20 ng/mL and that was completed within 15 min. A close inspection of 19 Monascus-fermented foods by cdELISA confirmed that 14 were contaminated with citrinin at levels from 28.6⁻9454 ng/g. Further analysis with the immunostrip is consistent with those results obtained using cdELISA. Both means are sensitive enough for the rapid examination of CTN in Monascus-fermented food products.
Keywords: Citrinin; ELISA; Monascus fermented food; gold nanoparticle immunochromatographic strip.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Carboxyl-Functionalized, Europium Nanoparticle-Based Fluorescent Immunochromatographic Assay for Sensitive Detection of Citrinin in Monascus Fermented Food.Toxins (Basel). 2019 Oct 17;11(10):605. doi: 10.3390/toxins11100605. Toxins (Basel). 2019. PMID: 31627364 Free PMC article.
-
Pilot production of a sensitive ELISA kit and an immunochromatographic strip for rapid detecting citrinin in fermented rice.RSC Adv. 2022 Jul 8;12(31):19981-19989. doi: 10.1039/d2ra02823a. eCollection 2022 Jul 6. RSC Adv. 2022. PMID: 35865211 Free PMC article.
-
Sensitive enzyme-linked immunosorbent assay and rapid one-step immunochromatographic strip for fumonisin B1 in grain-based food and feed samples.J Sci Food Agric. 2010 Apr 30;90(6):1020-6. doi: 10.1002/jsfa.3911. J Sci Food Agric. 2010. PMID: 20355142
-
Toxicological properties of citrinin.Arh Hig Rada Toksikol. 2009 Dec;60(4):457-64. doi: 10.2478/10004-1254-60-2009-1992. Arh Hig Rada Toksikol. 2009. PMID: 20061247 Review.
-
Occurrence, Risk Implications, Prevention and Control of CIT in Monascus Cheese: A Review.J Agric Food Chem. 2024 May 1;72(17):9567-9580. doi: 10.1021/acs.jafc.4c00588. Epub 2024 Apr 16. J Agric Food Chem. 2024. PMID: 38627202 Review.
Cited by
-
Carboxyl-Functionalized, Europium Nanoparticle-Based Fluorescent Immunochromatographic Assay for Sensitive Detection of Citrinin in Monascus Fermented Food.Toxins (Basel). 2019 Oct 17;11(10):605. doi: 10.3390/toxins11100605. Toxins (Basel). 2019. PMID: 31627364 Free PMC article.
-
Development of a Rapid Gold Nanoparticle Immunochromatographic Strip Based on the Nanobody for Detecting 2,4-DichloRophenoxyacetic Acid.Biosensors (Basel). 2022 Jan 30;12(2):84. doi: 10.3390/bios12020084. Biosensors (Basel). 2022. PMID: 35200344 Free PMC article.
-
The Effect of Blue Light on the Production of Citrinin in Monascus purpureus M9 by Regulating the mraox Gene through lncRNA AOANCR.Toxins (Basel). 2019 Sep 13;11(9):536. doi: 10.3390/toxins11090536. Toxins (Basel). 2019. PMID: 31540336 Free PMC article.
-
Development of Real-Time Immuno-PCR Based on Phage Displayed an Anti-Idiotypic Nanobody for Quantitative Determination of Citrinin in Monascus.Toxins (Basel). 2019 Sep 30;11(10):572. doi: 10.3390/toxins11100572. Toxins (Basel). 2019. PMID: 31575068 Free PMC article.
-
Development of an Enzyme-Linked Immunosorbent Assay Based on a Monoclonal Antibody for the Rapid Detection of Citrinin in Wine.Foods. 2024 Dec 25;14(1):27. doi: 10.3390/foods14010027. Foods. 2024. PMID: 39796317 Free PMC article.
References
-
- Liao C.D., Chen Y.C., Lin H.Y., Chiueh L.C., Shih D.Y.C. Incidence of citrinin in red yeast rice and various commercial Monascus products in Taiwan from 2009 to 2012. Food Control. 2014;38:178–183. doi: 10.1016/j.foodcont.2013.10.016. - DOI
-
- IARC . Citrinin. IARC Monograph 40. IARC; Lyons, France: 1986. pp. 67–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

