Immunogenicity of a Tripartite Cell Penetrating Peptide Containing a MUC1 Variable Number of Tandem Repeat (VNTR) and A T Helper Epitope
- PMID: 30200528
- PMCID: PMC6225367
- DOI: 10.3390/molecules23092233
Immunogenicity of a Tripartite Cell Penetrating Peptide Containing a MUC1 Variable Number of Tandem Repeat (VNTR) and A T Helper Epitope
Abstract
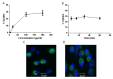
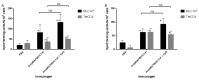
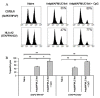
Peptide-based vaccines for cancer have many advantages however, for optimization these immunogens should incorporate peptide epitopes that induce CD8, as well as CD4 responses, antibody and long term immunity. Cell penetrating peptides (CPP) with a capacity of cytosolic delivery have been used to deliver antigenic peptides and proteins to antigen presenting cells to induce cytotoxic T cell, helper T cell and humoral responses in mice. For this study, a tripartite CPP including a mucin 1 (MUC1) variable number of tandem repeat (VNTR) containing multiple T cell epitopes and tetanus toxoid universal T helper epitope peptide (tetCD4) was synthesised (AntpMAPMUC1tet) and immune responses investigated in mice. Mice vaccinated with AntpMAPMUC1tet + CpG show enhanced antigen-specific interferon-gamma (IFN-γ) and IL-4 T cell responses compared with AntpMAPMUC1tet vaccination alone and induced a Th1 response, characterised by a higher ratio of IgG2a antibody/IgG1 antibodies. Furthermore, vaccination generated long term MUC1-specific antibody and T cell responses and delayed growth of MUC1+ve tumours in mice. This data demonstrates the efficient delivery of branched multiple antigen peptides incorporating CPP and that the addition of CpG augments immune responses.
Keywords: Mucin 1; TLR agonist; antigen delivery; immunogenicity; immunotherapy; membrane penetrating peptide; membrane translocating peptide; multiple antigen peptide; penetratin; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Comparative Immunogenicity of a Cytotoxic T Cell Epitope Delivered by Penetratin and TAT Cell Penetrating Peptides.Molecules. 2015 Aug 3;20(8):14033-50. doi: 10.3390/molecules200814033. Molecules. 2015. PMID: 26247926 Free PMC article.
-
T cells recognize PD(N/T)R motif common in a variable number of tandem repeat and degenerate repeat sequences of MUC1.Int Immunopharmacol. 2005 Feb;5(2):315-30. doi: 10.1016/j.intimp.2004.10.004. Int Immunopharmacol. 2005. PMID: 15652762
-
Definition of MHC-restricted CTL epitopes from non-variable number of tandem repeat sequence of MUC1.Vaccine. 2000 Apr 3;18(19):2059-71. doi: 10.1016/s0264-410x(99)00515-0. Vaccine. 2000. PMID: 10706970
-
Tecemotide: an antigen-specific cancer immunotherapy.Hum Vaccin Immunother. 2014;10(11):3383-93. doi: 10.4161/hv.29836. Hum Vaccin Immunother. 2014. PMID: 25483673 Free PMC article. Review.
-
Immunology of O-glycosylated proteins: approaches to the design of a MUC1 glycopeptide-based tumor vaccine.Curr Protein Pept Sci. 2006 Aug;7(4):307-15. doi: 10.2174/138920306778018034. Curr Protein Pept Sci. 2006. PMID: 16918445 Review.
Cited by
-
Recent Advances in Cell Penetrating Peptide-Based Anticancer Therapies.Molecules. 2019 Mar 7;24(5):927. doi: 10.3390/molecules24050927. Molecules. 2019. PMID: 30866424 Free PMC article. Review.
-
Cell-penetrating Peptides: Efficient Vectors for Vaccine Delivery.Curr Drug Deliv. 2019;16(5):430-443. doi: 10.2174/1567201816666190123120915. Curr Drug Deliv. 2019. PMID: 30760185 Free PMC article. Review.
-
Advances and prospects of cell-penetrating peptides in tumor immunotherapy.Sci Rep. 2025 Jan 27;15(1):3392. doi: 10.1038/s41598-025-86130-8. Sci Rep. 2025. PMID: 39870681 Free PMC article. Review.
-
TAT for Enzyme/Protein Delivery to Restore or Destroy Cell Activity in Human Diseases.Life (Basel). 2021 Sep 6;11(9):924. doi: 10.3390/life11090924. Life (Basel). 2021. PMID: 34575072 Free PMC article. Review.
-
Cell-penetrating peptides enhance peptide vaccine accumulation and persistence in lymph nodes to drive immunogenicity.Proc Natl Acad Sci U S A. 2022 Aug 9;119(32):e2204078119. doi: 10.1073/pnas.2204078119. Epub 2022 Aug 1. Proc Natl Acad Sci U S A. 2022. PMID: 35914154 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

