Fatty Acid Lingual Application Activates Gustatory and Reward Brain Circuits in the Mouse
- PMID: 30200577
- PMCID: PMC6163273
- DOI: 10.3390/nu10091246
Fatty Acid Lingual Application Activates Gustatory and Reward Brain Circuits in the Mouse
Abstract
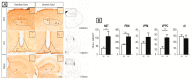
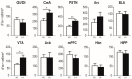
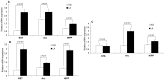
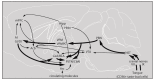
The origin of spontaneous preference for dietary lipids in humans and rodents is debated, though recent compelling evidence has shown the existence of fat taste that might be considered a sixth taste quality. We investigated the implication of gustatory and reward brain circuits, triggered by linoleic acid (LA), a long-chain fatty acid. The LA was applied onto the circumvallate papillae for 30 min in conscious C57BL/6J mice, and neuronal activation was assessed using c-Fos immunohistochemistry. By using real-time reverse transcription polymerase chain reaction (RT-qPCR), we also studied the expression of mRNA encoding brain-derived neurotrophic factor (BDNF), Zif-268, and Glut-1 in some brain areas of these animals. LA induced a significant increase in c-Fos expression in the nucleus of solitary tract (NST), parabrachial nucleus (PBN), and ventroposterior medialis parvocellularis (VPMPC) of the thalamus, which are the regions known to be activated by gustatory signals. LA also triggered c-Fos expression in the central amygdala and ventral tegmental area (VTA), involved in food reward, in conjunction with emotional traits. Interestingly, we noticed a high expression of BDNF, Zif-268, and Glut-1 mRNA in the arcuate nucleus (Arc) and hippocampus (Hipp), where neuronal activation leads to memory formation. Our study demonstrates that oral lipid taste perception might trigger the activation of canonical gustatory and reward pathways.
Keywords: BDNF; Glut-1; Zif-268; c-Fos; fat taste; gustation; hedonic; linoleic acid.
Conflict of interest statement
All the authors declare that they have nothing to disclose.
Figures
Similar articles
-
The gustatory pathway is involved in CD36-mediated orosensory perception of long-chain fatty acids in the mouse.FASEB J. 2008 May;22(5):1458-68. doi: 10.1096/fj.07-8415com. Epub 2007 Dec 27. FASEB J. 2008. PMID: 18162488
-
Expression of Fos during sham sucrose intake in rats with central gustatory lesions.Am J Physiol Regul Integr Comp Physiol. 2008 Sep;295(3):R751-63. doi: 10.1152/ajpregu.90344.2008. Epub 2008 Jul 16. Am J Physiol Regul Integr Comp Physiol. 2008. PMID: 18635449 Free PMC article.
-
Cell mechanisms of gustatory lipids perception and modulation of the dietary fat preference.Biochimie. 2014 Dec;107 Pt A:11-4. doi: 10.1016/j.biochi.2014.06.018. Epub 2014 Jul 2. Biochimie. 2014. PMID: 24997404 Review.
-
Sweet and bitter taste stimuli activate VTA projection neurons in the parabrachial nucleus.Brain Res. 2019 Jul 1;1714:99-110. doi: 10.1016/j.brainres.2019.02.027. Epub 2019 Feb 23. Brain Res. 2019. PMID: 30807736 Free PMC article.
-
Dual separate pathways for sensory and hedonic aspects of taste.Brain Res Bull. 2004 Jan 15;62(4):271-83. doi: 10.1016/j.brainresbull.2003.10.004. Brain Res Bull. 2004. PMID: 14709342 Review.
Cited by
-
Hypothalamic Fatty Acids and Ketone Bodies Sensing and Role of FAT/CD36 in the Regulation of Food Intake.Front Physiol. 2019 Aug 14;10:1036. doi: 10.3389/fphys.2019.01036. eCollection 2019. Front Physiol. 2019. PMID: 31474875 Free PMC article. Review.
-
Novel GPR120 agonist TUG891 modulates fat taste perception and preference and activates tongue-brain-gut axis in mice.J Lipid Res. 2020 Feb;61(2):133-142. doi: 10.1194/jlr.RA119000142. Epub 2019 Dec 5. J Lipid Res. 2020. PMID: 31806728 Free PMC article.
-
The effect of maternal period nutritional status on oro-sensorial fat perception and taste preference in rats.Mol Cell Biochem. 2023 Dec;478(12):2861-2873. doi: 10.1007/s11010-023-04703-5. Epub 2023 Mar 21. Mol Cell Biochem. 2023. PMID: 36943662
-
A systematic review of the biological mediators of fat taste and smell.Physiol Rev. 2023 Jan 1;103(1):855-918. doi: 10.1152/physrev.00061.2021. Epub 2022 Sep 15. Physiol Rev. 2023. PMID: 36409650 Free PMC article.
-
Exogenous oral application of PYY and exendin-4 impacts upon taste-related behavior and taste perception in wild-type mice.Neuropharmacology. 2025 Jul 1;272:110408. doi: 10.1016/j.neuropharm.2025.110408. Epub 2025 Mar 12. Neuropharmacology. 2025. PMID: 40086622
References
-
- Hess M.A. Resetting the American table—Creating a new alliance of taste and health. J. Am. Diet. Assoc. 1991;91:228–230. - PubMed
-
- Ozdener M.H., Subramaniam S., Sundaresan S., Sery O., Hashimoto T., Asakawa Y., Besnard P., Abumrad N.A., Khan N.A. CD36-and GPR120-mediated Ca2+ signaling in human taste bud cells mediates differential responses to fatty acids and is altered in obese mice. Gastroenterology. 2014;146:995–1005. doi: 10.1053/j.gastro.2014.01.006. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

