Pharmacokinetics and Anti-Gastric Ulceration Activity of Oral Administration of Aceclofenac and Esomeprazole in Rats
- PMID: 30200587
- PMCID: PMC6160962
- DOI: 10.3390/pharmaceutics10030152
Pharmacokinetics and Anti-Gastric Ulceration Activity of Oral Administration of Aceclofenac and Esomeprazole in Rats
Abstract
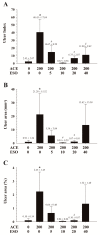
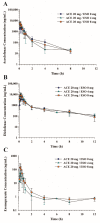
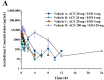
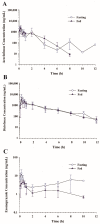
This study examined the effects of esomeprazole on aceclofenac pharmacokinetics and gastrointestinal complications in rats. Aceclofenac alone, or in combination with esomeprazole, was orally administered to male Sprague-Dawley rats. Plasma concentrations of aceclofenac, its major metabolite diclofenac, and esomeprazole were simultaneously determined by a novel liquid chromatography-tandem mass spectrometry method. Gastrointestinal damage was determined by measuring ulcer area and ulcer lesion index of the stomach. Oral administration of aceclofenac induced significant gastric ulceration, which was inhibited by esomeprazole administration. Following concurrent administration of aceclofenac and esomeprazole, overall pharmacokinetic profiles of aceclofenac and metabolic conversion to diclofenac were unaffected by esomeprazole. Aceclofenac metabolism and pharmacokinetics were not subject to significant food effects, whereas bioavailability of esomeprazole decreased in fed compared to fasting conditions. In contrast, the pharmacokinetics of aceclofenac and esomeprazole were significantly altered by different dosing vehicles. These results suggest that co-administration of esomeprazole with aceclofenac may reduce aceclofenac-induced gastrointestinal complications without significant pharmacokinetic interactions. The optimal combination and clinical significance of the benefits of the combination of aceclofenac and esomeprazole need to be further evaluated.
Keywords: aceclofenac; diclofenac; esomeprazole; gastric ulcer; pharmacokinetics.
Conflict of interest statement
The authors declare no conflict of interest. Y.-W.C., S.-M.C., K.-Y.N., and W.-H.K. are full-time employees of Korea United Pharm. Inc. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures

References
-
- Batlle-Gualda E., Figueroa M., Ivorra J., Raber A. The efficacy and tolerability of aceclofenac in the treatment of patients with ankylosing spondylitis: A multicenter controlled clinical trial. Aceclofenac indomethacin study group. J. Rheumatol. 1996;23:1200–1206. - PubMed
-
- Movilia P.G. Evaluation of the analgesic activity and tolerability of aceclofenac in the treatment of post-episiotomy pain. Drugs Exp. Clin. Res. 1989;15:47–51. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

