Implications of Pyran Cyclization and Pterin Conformation on Oxidized Forms of the Molybdenum Cofactor
- PMID: 30200760
- PMCID: PMC6542470
- DOI: 10.1021/jacs.8b05777
Implications of Pyran Cyclization and Pterin Conformation on Oxidized Forms of the Molybdenum Cofactor
Abstract
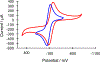
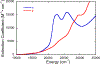
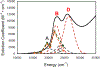
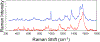
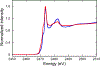
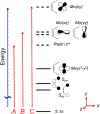
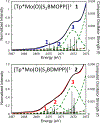
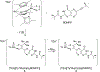
The large family of mononuclear molybdenum and tungsten enzymes all possess the special ligand molybdopterin (MPT), which consists of a metal-binding dithiolene chelate covalently bound to a pyranopterin group. MPT pyran cyclization/scission processes have been proposed to modulate the reactivity of the metal center during catalysis. We have designed several small-molecule models for the Mo-MPT cofactor that allow detailed investigation into how pyran cyclization modulates electronic communication between the dithiolene and pterin moieties and how this cyclization alters the electronic environment of the molybdenum catalytic site. Using a combination of cyclic voltammetry, vibrational spectroscopy (FT-IR and rR), electronic absorption spectroscopy, and X-ray absorption spectroscopy, distinct changes in the Mo≡O stretching frequency, Mo(V/IV) reduction potential, and electronic structure across the pterin-dithiolene ligand are observed as a function of pyran ring closure. The results are significant, for they reveal that a dihydropyranopterin is electronically coupled into the Mo-dithiolene group due to a coplanar conformation of the pterin and dithiolene units, providing a mechanism for the electron-deficient pterin to modulate the Mo environment. A spectroscopic signature identified for the dihydropyranopterin-dithiolene ligand on Mo is a strong dithiolene → pterin charge transfer transition. In the absence of a pyran group bridge between pterin and dithiolene, the pterin rotates out of plane, largely decoupling the system. The results support a hypothesis that pyran cyclization/scission processes in MPT may function as a molecular switch to electronically couple and decouple the pterin and dithiolene to adjust the redox properties in certain pyranopterin molybdenum enzymes.
Figures
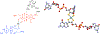
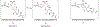
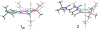
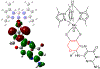


References
-
- Kirk ML, Spectroscopic and Electronic Structure Studies of Mo Model Compounds and Enzymes In Molybdenum and Tungsten Enzymes: Spectroscopic and Theoretical Investigations, The Royal Society of Chemistry: Cambridge, UK, 2016.
-
- Kirk ML; Stein B, The Molybdenum Enzymes In Comprehensive Inorganic Chemistry II (Second Edition), Editors-in-Chief: Jan R; Kenneth P, Eds. Elsevier: Amsterdam, 2013; pp 263–293.
-
- Maia LB; Moura I; Moura JJG, Molybdenum and Tungsten-Containing Enzymes: An Overview. 2017; Vol. 5, p 1–80.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

