Predictors for false-negative QuantiFERON-TB Gold assay results in patients with extrapulmonary tuberculosis
- PMID: 30200884
- PMCID: PMC6131843
- DOI: 10.1186/s12879-018-3344-x
Predictors for false-negative QuantiFERON-TB Gold assay results in patients with extrapulmonary tuberculosis
Abstract
Backgrounds: Extrapulmonary tuberculosis (EPTB) is a heterogeneous disease, and diagnosis is sometimes difficult. We investigated the diagnostic performance of the QuantiFERON-TB Gold assay (QFT-GIT) according to sites of EPTB and predictors for false-negative QFT-GIT results.
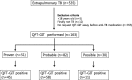
Methods: A total of 2176 patients were registered with active TB from January 2012 to December 2016 in Seoul St. Mary's Hospital, a 1200-bed tertiary teaching hospital in Seoul, Korea. We retrospectively reviewed the medical records of 163 EPTB patients who underwent QFT-GIT.
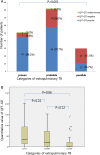
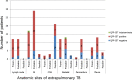
Results: False negative QFT-GIT results were found in 28.8% (95% CI 0.22-0.36) of patients with EPTB. In the proven TB group, negative QFT-GIT results were found in 28.6% (95% CI 0.04-0.71) of pleural, 8.3% 0.002-0.38of lymph node, 8.3% (95% CI 0.002-0.38) of skeletal and 5.8% (95% CI 0.001-0.28) of gastrointestinal TB cases. Among probable TB cases, QFT-GIT negative results were identified in 46.2% (95% CI 0.19-0.75) of skeletal, 33.3% (95% CI 10-0.65) of pericardial, 30.8% (95% CI 0.09-0.61) of pleural and 17.2% (95% CI 0.10-0.56) of gastrointestinal TB cases. In the possible TB cases, central nervous system TB (n = 21) was most frequent, and 66.7% (95% CI 0.43-0.85) of those showed QFT-GIT negative results. By multivariate analysis, possible TB was independently associated with false-negative QFT-GIT results (OR 4.92, 95% CI 1.51-16.06, p = 0.008).
Conclusions: Prudent interpretation of QFT-GIT results might be needed according to anatomic site of involvement and diagnostic criteria in patients with high suspicion of EPTB.
Keywords: Extrapulmonary tuberculosis; False negative; IFN-gamma release assay.
Conflict of interest statement
Ethics approval and consent to participate
The study was approved by the Institutional Review Board of Seoul St. Mary’s Hospital (KC18RESI0142). The study was a secondary data analysis and did not involve contact with individual patients so consent to participate was not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- WHO. Global tuberculosis report 2016. Geneva: World Health Organization; 2016. http://www.who.int/tb/publications/global_report/en/. Accessed 1 Jan 2018.
-
- Korean Statistical Information Service NHIS. 2018.http://kosis.kr/index/index.do.
-
- Sester M, Sotgiu G, Lange C, Giehl C, Girardi E, Migliori GB, Bossink A, Dheda K, Diel R, Dominguez J, et al. Interferon-gamma release assays for the diagnosis of active tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2011;37(1):100–111. doi: 10.1183/09031936.00114810. - DOI - PubMed
-
- Bennett JE, Raphael D, Blaser MJ. Mandell Douglas, and Bennett's principles and practice of infectious diseases. 8. Canada: Saunders; 2015.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

