CRITICS-II: a multicentre randomised phase II trial of neo-adjuvant chemotherapy followed by surgery versus neo-adjuvant chemotherapy and subsequent chemoradiotherapy followed by surgery versus neo-adjuvant chemoradiotherapy followed by surgery in resectable gastric cancer
- PMID: 30200910
- PMCID: PMC6131797
- DOI: 10.1186/s12885-018-4770-2
CRITICS-II: a multicentre randomised phase II trial of neo-adjuvant chemotherapy followed by surgery versus neo-adjuvant chemotherapy and subsequent chemoradiotherapy followed by surgery versus neo-adjuvant chemoradiotherapy followed by surgery in resectable gastric cancer
Abstract
Background: Although radical surgery remains the cornerstone of cure in resectable gastric cancer, survival remains poor. Current evidence-based (neo)adjuvant strategies have shown to improve outcome, including perioperative chemotherapy, postoperative chemoradiotherapy and postoperative chemotherapy. However, these regimens suffer from poor patient compliance, particularly in the postoperative phase of treatment. The CRITICS-II trial aims to optimize preoperative treatment by comparing three treatment regimens: (1) chemotherapy, (2) chemotherapy followed by chemoradiotherapy and (3) chemoradiotherapy.
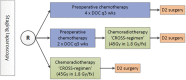
Methods: In this multicentre phase II non-comparative study, patients with clinical stage IB-IIIC (TNM 8th edition) resectable gastric adenocarcinoma are randomised between: (1) 4 cycles of docetaxel+oxaliplatin+capecitabine (DOC), (2) 2 cycles of DOC followed by chemoradiotherapy (45Gy in combination with weekly paclitaxel and carboplatin) or (3) chemoradiotherapy. Primary endpoint is event-free survival, 1 year after randomisation (events are local and/or regional recurrence or progression, distant recurrence, or death from any cause). Secondary endpoints include: toxicity, surgical outcomes, percentage radical (R0) resections, pathological tumour response, disease recurrence, overall survival, and health related quality of life. Exploratory endpoints include translational studies on predictive and prognostic biomarkers.
Discussion: The aim of this study is to select the most promising among three preoperative treatment arms in patients with resectable gastric adenocarcinoma. This treatment regimen will subsequently be compared with the standard therapy in a phase III trial.
Trial registration: clinicaltrials.gov NCT02931890 ; registered 13 October 2016. Date of first enrolment: 21 December 2017.
Keywords: Chemoradiotherapy; Chemotherapy; Gastric cancer; Preoperative treatment; Resectable; Surgery.
Conflict of interest statement
Ethics approval and consent to participate
This study is conducted in agreement with either the Declaration of Helsinki or the laws and regulations of the country, whichever provides the greatest protection of the patient. The protocol has been written, and the study is conducted according to the ICH Harmonized Tripartite Guideline for Good Clinical Practice. The CRITICS-II trial has been approved by the medical ethics committee of the Antoni van Leeuwenhoek, Amsterdam. Patients receive both oral and written information about the study. Written informed consent will be obtained before any study procedures. Most recent protocol version is version 3.1 and was approved by the medical ethics committee at 18 April 2018. The sponsor/investigator has a liability insurance that provides cover for manage to research subjects through injury or death caused by the study. The insurance applies to the damage that becomes apparent during the study or within 4 years after the end of the study.
Study ID Numbers:
NL55436.031.16 (Registry identifier: CCMO)
2015–004627-31 (EudraCT Number)
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- De Angelis R, Sant M, Coleman MP, Francisci S, Baili P, Pierannunzio D, et al. Cancer survival in Europe 1999-2007 by country and age: results of EUROCARE--5-a population-based study. Lancet Oncol. 2014;15(1):23–34. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical