Protocol for a clinical trial of text messaging in addition to standard care versus standard care alone in prevention of type 2 diabetes through lifestyle modification in India and the UK
- PMID: 30200935
- PMCID: PMC6131868
- DOI: 10.1186/s12902-018-0293-8
Protocol for a clinical trial of text messaging in addition to standard care versus standard care alone in prevention of type 2 diabetes through lifestyle modification in India and the UK
Abstract
Background: Type 2 diabetes is a serious clinical problem in both India and the UK. Adoption of a healthy lifestyle through dietary and physical activity modification can help prevent type 2 diabetes. However, implementing lifestyle modification programmes to high risk groups is expensive and alternative cheaper methods are needed. We are using a short messaging service (SMS) programme in our study as a tool to provide healthy lifestyle advice and an aid to motivation. The aim of the study is to assess the efficacy and user acceptability of text messaging employed in this way for people with pre-diabetes (HbA1c 6.0% to ≤6.4%; 42-47 mmol/mol) in the UK and India.
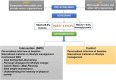
Methods/design: This is a randomised, controlled trial with participants followed up for 2 years. After being screened and receiving a structured education programme for prediabetes, participants are randomised to a control or intervention group. In the intervention group, text messages are delivered 2-3 times weekly and contain educational, motivational and supportive content on diet, physical activity, lifestyle and smoking. The control group undergoes monitoring only. In India, the trial involves 5 visits after screening (0, 6, 12, 18 and 24 months). In the UK there are 4 visits after screening (0, 6, 12 and 24 months). Questionnaires (EQ-5D, RPAQ, Transtheoretical Model of Behavioural Change, and food frequency (UK)/24 h dietary recall (India)) and physical activity monitors (Actigraph GT3X+ accelerometers) are assessed at baseline and all follow-up visits. The SMS acceptability questionnaires are evaluated in all follow-up visits. The primary outcome is progression to type 2 diabetes as defined by an HbA1c of 6.5% or over(India) and by any WHO criterion(UK). Secondary outcomes are the changes in body weight, body mass index, waist circumference, blood pressure, fasting plasma glucose; lipids; proportion of participants achieving HbA1c ≤6.0%; HOMA-IR; HOMA-β; acceptability of SMS; dietary parameters; physical activity and quality of life.
Discussion: The study is designed to assess the efficacy of tailored text messaging in addition to standard lifestyle advice to reduce the progression from prediabetes to type 2 diabetes in the two different countries.
Trial registration: ClinicalTrials.gov ; NCT01570946 , 4th April 2012 (India); NCT01795833 , 21st February 2013 (UK).
Keywords: Diabetes prevention; HbA1c; Prediabetes; Randomised controlled trial; Short messaging service.
Conflict of interest statement
Ethics approval and consent to participate
Ethics approvals were received from Westminster Ethics Committee (12/LO/1322) in the UK and from the Ethics Committee of the India Diabetes Research Foundation (ECR/254/Inst/TN/2013) in India. Study protocol amendments are reviewed for approval by ethics committees when necessary. Written consent is obtained from each participant after full explanation, an information leaflet offered and time allowed for consideration. The right of the participant to refuse to participate, or continue participating, without giving reasons and without prejudicing further treatment is respected. The Chief Investigator in each country ensures that participant confidentiality is respected and local data protection requirements are met.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
A pragmatic and scalable strategy using mobile technology to promote sustained lifestyle changes to prevent type 2 diabetes in India and the UK: a randomised controlled trial.Diabetologia. 2020 Mar;63(3):486-496. doi: 10.1007/s00125-019-05061-y. Epub 2020 Jan 9. Diabetologia. 2020. PMID: 31919539 Free PMC article. Clinical Trial.
-
Behavioural interventions to promote physical activity in a multiethnic population at high risk of diabetes: PROPELS three-arm RCT.Health Technol Assess. 2021 Dec;25(77):1-190. doi: 10.3310/hta25770. Health Technol Assess. 2021. PMID: 34995176 Clinical Trial.
-
Effectiveness of mobile phone messaging in prevention of type 2 diabetes by lifestyle modification in men in India: a prospective, parallel-group, randomised controlled trial.Lancet Diabetes Endocrinol. 2013 Nov;1(3):191-8. doi: 10.1016/S2213-8587(13)70067-6. Epub 2013 Sep 11. Lancet Diabetes Endocrinol. 2013. PMID: 24622367 Clinical Trial.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Effects of dietary and physical activity interventions on the risk of type 2 diabetes in South Asians: meta-analysis of individual participant data from randomised controlled trials.Diabetologia. 2019 Aug;62(8):1337-1348. doi: 10.1007/s00125-019-4905-2. Epub 2019 Jun 15. Diabetologia. 2019. PMID: 31201437 Review.
Cited by
-
Cut-off Value of Random Blood Glucose among Asian Indians for Preliminary Screening of Persons with Prediabetes and Undetected Type 2 Diabetes Defined by the Glycosylated Haemoglobin Criteria.J Diabetes Clin Res. 2019;1(2):53-58. doi: 10.33696/diabetes.1.009. J Diabetes Clin Res. 2019. PMID: 32133459 Free PMC article.
-
The Development of Text Messages to Support People at Risk of Diabetes in Low-Resourced Communities: The South African Diabetes Prevention Programme.Nutrients. 2023 Nov 5;15(21):4692. doi: 10.3390/nu15214692. Nutrients. 2023. PMID: 37960345 Free PMC article.
-
A pragmatic and scalable strategy using mobile technology to promote sustained lifestyle changes to prevent type 2 diabetes in India and the UK: a randomised controlled trial.Diabetologia. 2020 Mar;63(3):486-496. doi: 10.1007/s00125-019-05061-y. Epub 2020 Jan 9. Diabetologia. 2020. PMID: 31919539 Free PMC article. Clinical Trial.
References
-
- Public Health England. Quality and Outcome Framework: Diabetes Prevalence: Public Health England; [Available from: https://fingertips.phe.org.uk/profile/diabetes-ft.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous