Progressive pseudorheumatoid dysplasia with new-found gene mutation of Wntl inducible signaling pathway protein 3
- PMID: 30200995
- PMCID: PMC6131911
- DOI: 10.1186/s12969-018-0272-7
Progressive pseudorheumatoid dysplasia with new-found gene mutation of Wntl inducible signaling pathway protein 3
Abstract
Background: As one kind of osteochondrodysplasia, progressive pseudorheumatoid dysplasia (PPD) is also known as spondyloepiphyseal dysplasia tarda with progressive arthropathy or arthropathy progressive pseudorheumatoid of childhood. PPD is a very rare disease, especially in China, and has an estimated prevalence of 1/1000000 due to lacking definite prevalence survey. It is an autosomal recessive disorder caused by gene mutation of Wntl inducible signaling pathway protein 3 (WISP3). Its basic pathological change is persistent degeneration and loss of articular cartilage in multiple joints. Its clinical appearances include bone enlargement, platyspondyly, irregular endplate, secondary osteoarthritis, extensive osteoporosis, joint rigidity and function loss. Clinical diagnosis of PPD is made based on clinical appearance and imaging examinations, whereas its definite diagnosis depends on gene sequencing. PPD has no severe effect on life span, but causes high disability rate and very poor prognosis. There are only case reports with limited information in China.
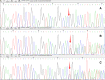
Case presentation: One female patient was diagnosed as PPD and secondary osteoarthritis. She had typical clinical appearance and imaging examinations, and received individualized therapeutic regimens. She had a gene mutation (c.72delT, p.T24TfsX4) of WISP3. This gene mutation has not been reported by previous literatures and included in Single Nucleotide Polymorphism Database.
Conclusions: As the first time, this paper reported a patient with PPD caused by new-found gene mutation (c.72delT, p.T24TfsX4) of WISP3.
Keywords: Progressive pseudorheumatoid dysplasia; Wntl inducible signaling pathway protein 3.
Conflict of interest statement
Ethics approval and consent to participate
The study protocol was approved by Ethics Committee of Hainan Branch of Chinese People’s Liberation Army General Hospital (Sanya, China). This participant provided written informed consent to be included in the study.
Consent for publication
Written consent for publication was obtained from this patient.
Competing interests
The authors declare that they have no conflict of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Garcia Segarra N, Mittaz L, Campos-Xavier AB, Bartels CF, Tuysuz B, Alanay Y, et al. The diagnostic challenge of progressive pseudorheumatoid dysplasia (PPRD): a review of clinical features, radiographic features, and WISP3 mutations in 63 affected individuals. Am J Med Genet C Semin Med Genet. 2012;160:217–229. doi: 10.1002/ajmg.c.31333. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

