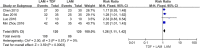Lamivudine plus tenofovir combination therapy versus lamivudine monotherapy for HBV/HIV coinfection: a meta-analysis
- PMID: 30201035
- PMCID: PMC6130076
- DOI: 10.1186/s12985-018-1050-3
Lamivudine plus tenofovir combination therapy versus lamivudine monotherapy for HBV/HIV coinfection: a meta-analysis
Abstract
Background: Currently, there is no consensus on the efficacy and safety of lamivudine (LAM) plus tenofovir disoproxil fumarate (TDF) combination therapy versus lamivudine monotherapy in HBV/HIV coinfected patients.
Methods: A comprehensive literature search was performed in English and Chinese databases. Both relevant dichotomous and continuous variables were extracted, and the combined outcomes were expressed as a risk ratio (RR) or a standard mean difference (SMD).
Results: Eleven eligible studies were included in our analysis. For HBV-relevant outcomes, the proportion of patients with undetectable HBV, the rates of serum alanine aminotransferase (ALT) normalization and hepatitis B e antigen (HBeAg) loss were higher in the combination therapy group than the monotherapy group (RR = 1.42, 95% CI: 1.14-1.76, P = 0.002; RR = 1.36, 95% CI: 1.17-1.58, P < 0.0001; RR = 2.74, 95% CI: 1.20-6.22, P = 0.02). In addition, the rate of HIV RNA-negative conversion was higher in the combination therapy group than the monotherapy group (RR = 1.26, 95% CI: 1.11-1.42, P = 0.0003).
Conclusion: LAM plus TDF combination therapy was more efficacious than LAM monotherapy in HBV/HIV coinfected patients. As time passes, this difference becomes more pronounced.
Keywords: HBV/HIV coinfection; Lamivudine; Tenofovir.
Conflict of interest statement
Ethics approval and consent to participate
not applicable.
Consent for publication
not applicable.
Competing interests
The authors’ declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Diederich WE, Steuber H. Therapy of viral infections. Topics Med Chem. 2015;2:35–56.
-
- Fischer J. Analogue-based drug discovery. Chemistry International -- Newsmagazine for IUPAC. 2010;32:12–15.
-
- Sharp M, Interpharma D, Sharp M, Interpharma D, Celle L, Cloud S. Sharp M. WHO Model List of Essential Medicines: Interpharma D; 2011.
Publication types
MeSH terms
Substances
Grants and funding
- 2017ZX10202203-007/National Science and Technology Major Project of China/International
- 2017ZX10202203-008/National Science and Technology Major Project of China/International
- cstc2012jjA10064/Natural Science Foundation Project of CQCSTC/International
- cstc2013jcyjA10060/Natural Science Foundation Project of CQCSTC/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials