Sex-specific differences in hepatic steatosis in obese spontaneously hypertensive (SHROB) rats
- PMID: 30201044
- PMCID: PMC6131947
- DOI: 10.1186/s13293-018-0202-x
Sex-specific differences in hepatic steatosis in obese spontaneously hypertensive (SHROB) rats
Abstract
Background: Patients with metabolic syndrome, who are characterized by co-existence of insulin resistance, hypertension, hyperlipidemia, and obesity, are also prone to develop non-alcoholic fatty liver disease (NAFLD). Although the prevalence and severity of NAFLD is significantly greater in men than women, the mechanisms by which gender modulates the pathogenesis of hepatic steatosis are poorly defined. The obese spontaneously hypertensive (SHROB) rats represent an attractive model of metabolic syndrome without overt type 2 diabetes. Although pathological manifestation caused by the absence of a functional leptin receptor has been extensively studied in SHROB rats, it is unknown whether these animals elicited sex-specific differences in the development of hepatic steatosis.
Methods: We compared hepatic pathology in male and female SHROB rats. Additionally, we examined key biochemical and molecular parameters of signaling pathways linked with hyperinsulinemia and hyperlipidemia. Finally, using methods of quantitative polymerase chain reaction (qPCR) and western blot analysis, we quantified expression of 45 genes related to lipid biosynthesis and metabolism in the livers of male and female SHROB rats.
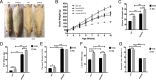
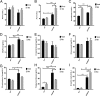
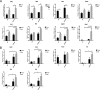
Results: We show that all SHROB rats developed hepatic steatosis that was accompanied by enhanced expression of SREBP1, SREBP2, ACC1, and FASN proteins. The livers of male rats also elicited higher induction of Pparg, Ppara, Slc2a4, Atox1, Skp1, Angptl3, and Pnpla3 mRNAs. In contrast, the livers of female SHROB rats elicited constitutively higher levels of phosphorylated JNK and AMPK and enhanced expression of Cd36.
Conclusion: Based on these data, we conclude that the severity of hepatic steatosis in male and female SHROB rats was mainly driven by increased de novo lipogenesis. Moreover, male and female SHROB rats also elicited differential severity of hepatic steatosis that was coupled with sex-specific differences in fatty acid transport and esterification.
Conflict of interest statement
Ethics approval and consent to participate
All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of University of Tennessee Health Science Center (UTHSC).
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures





References
-
- Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–495. doi: 10.1016/S0092-8674(00)81294-5. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

