Efficacy of topical tranexamic acid within a blood-saving programme for primary total hip arthroplasty: a pragmatic, open-label randomised study
- PMID: 30201083
- PMCID: PMC6214828
- DOI: 10.2450/2018.0133-18
Efficacy of topical tranexamic acid within a blood-saving programme for primary total hip arthroplasty: a pragmatic, open-label randomised study
Abstract
Background: Total hip arthroplasty entails considerable peri-operative blood loss, which may lead to acute post-operative anaemia and red blood cell transfusion. This study was aimed at assessing whether the addition of topical tranexamic acid to our ongoing blood-saving protocol for total hip arthroplasty was effective and safe.
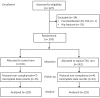
Materials and methods: A pragmatic, prospective, open-label randomised study of patients scheduled for total hip arthroplasty at a single centre was conducted. Consecutive patients were randomly assigned to receive topical tranexamic acid (2 g) at the end of surgery (tranexamic group, n=125) or not (control group, n=129). A restrictive transfusion protocol was applied. Outcome measures were red blood cell loss at 24 hours after surgery, in-hospital transfusion rate, and incidence of thromboembolic complications.
Results: Topical tranexamic acid was effective in reducing both red cell loss (mean difference: 138 mL [95% CI 87-189 mL]; p<0.001) in the 24h after surgery and in-hospital transfusion rates (12 vs 32.6%, for the tranexamic acid and control groups, respectively; p<0.001; relative risk=0.37 [95% CI 0.22-0.63]). However, relative red cell loss and transfusion rates were higher in females than in males, irrespectively of tranexamic acid use. The beneficial effect of tranexamic acid on transfusion was restricted to patients with pre-operative haemoglobin ≥13 g/dL (5.1 vs 24.8%; p<0.001). Topical tranexamic acid was well tolerated and no clinically apparent thromboembolic complications were witnessed.
Discussion: The use of topical tranexamic acid after hip arthroplasty reduced red cell loss and transfusion rates; the efficacy of this strategy may be improved by reinforcing both pre-operative haemoglobin optimisation and adherence to the practice of transfusing single units of red cells.
Conflict of interest statement
MM has received honoraria for lectures and/or consultancies from Vifor Pharma (Spain & Switzerland), Wellspect HealthCare (Sweden), Pharmacosmos (Denmark), Ferrer Pharma (Spain), CSL Behring (Germany), PharmaNutra (Italy) and Zambon (Spain).
The other Authors declare no conflicts of interest.
Figures

Comment in
-
The key role of tranexamic acid in Patient Blood Management programmes.Blood Transfus. 2018 Nov;16(6):471-472. doi: 10.2450/2018.0177-18. Blood Transfus. 2018. PMID: 30388070 Free PMC article. No abstract available.
References
-
- Rosencher N, Kerkkamp HE, Macheras G, et al. Orthopedic Surgery Transfusion Hemoglobin European Overview (OSTHEO) study: blood management in elective knee and hip arthroplasty in Europe. Transfusion. 2003;43:459–69. - PubMed
-
- Gombotz H, Rehak PH, Shander A, Hofmann A. The second Austrian benchmark study for blood use in elective surgery: results and practice change. Transfusion. 2014;54:2646–57. - PubMed
-
- Van der Linden P, Hardy JF. Implementation of patient blood management remains extremely variable in Europe and Canada: the NATA benchmark project: an observational study. Eur J Anaesthesiol. 2016;33:913–21. - PubMed
-
- Kotzé A, Carter LA, Scally AJ. Effect of a patient blood management programme on preoperative anaemia, transfusion rate, and outcome after primary hip or knee arthroplasty: a quality improvement cycle. Br J Anaesth. 2012;108:943–52. - PubMed
-
- Isbister JP, Shander A, Spahn DR, et al. Adverse blood transfusion outcomes: establishing causation. Transfus Med Rev. 2011;25:89–101. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
