The influence of the descending pain modulatory system on infant pain-related brain activity
- PMID: 30201093
- PMCID: PMC6133549
- DOI: 10.7554/eLife.37125
The influence of the descending pain modulatory system on infant pain-related brain activity
Abstract
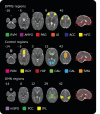
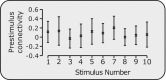
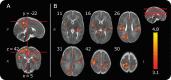
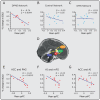
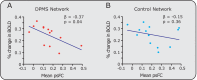
The descending pain modulatory system (DPMS) constitutes a network of widely distributed brain regions whose integrated function is essential for effective modulation of sensory input to the central nervous system and behavioural responses to pain. Animal studies demonstrate that young rodents have an immature DPMS, but comparable studies have not been conducted in human infants. In Goksan et al. (2015) we used functional MRI (fMRI) to show that pain-related brain activity in newborn infants is similar to that observed in adults. Here, we investigated whether the functional network connectivity strength across the infant DPMS influences the magnitude of this brain activity. FMRI scans were collected while mild mechanical noxious stimulation was applied to the infant's foot. Greater pre-stimulus functional network connectivity across the DPMS was significantly associated with lower noxious-evoked brain activity (p = 0.0004, r = -0.86, n = 13), suggesting that in newborn infants the DPMS may regulate the magnitude of noxious-evoked brain activity.
Keywords: brain; descending pain modulation; human; infant; neuroscience; pain.
© 2018, Goksan et al.
Conflict of interest statement
SG, LB, FM, ED, GH, CH, IT, RS No competing interests declared
Figures


References
Publication types
MeSH terms
Grants and funding
- 203139/Z/16/Z/Wellcome Trust/United Kingdom
- Senior Research Fellowship, 207457/Z/17/Z/Wellcome/International
- 207457/Z/17/Z/Wellcome Trust/United Kingdom
- Wellcome Centre for Integrative Neuroimaging, 203139/Z/16/Z/Wellcome/International
- Clinical Doctoral Fellowship/National Institute for Health Research/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

