Adamts10 inactivation in mice leads to persistence of ocular microfibrils subsequent to reduced fibrillin-2 cleavage
- PMID: 30201140
- PMCID: PMC8209899
- DOI: 10.1016/j.matbio.2018.09.004
Adamts10 inactivation in mice leads to persistence of ocular microfibrils subsequent to reduced fibrillin-2 cleavage
Abstract
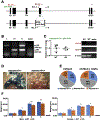
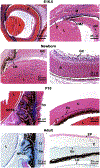
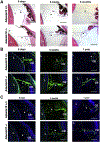
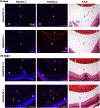
Mutations in the secreted metalloproteinase ADAMTS10 cause recessive Weill-Marchesani syndrome (WMS), comprising ectopia lentis, short stature, brachydactyly, thick skin and cardiac valve anomalies. Dominant WMS caused by FBN1 mutations is clinically similar and affects fibrillin-1 microfibrils, which are a major component of the ocular zonule. ADAMTS10 was previously shown to enhance fibrillin-1 assembly in vitro. Here, Adamts10 null mice were analyzed to determine the impact of ADAMTS10 deficiency on fibrillin microfibrils in vivo. An intragenic lacZ reporter identified widespread Adamts10 expression in the eye, musculoskeletal tissues, vasculature, skin and lung. Adamts10-/- mice had reduced viability on the C57BL/6 background, and although surviving mice were slightly smaller and had stiff skin, they lacked brachydactyly and cardiovascular defects. Ectopia lentis was not observed in Adamts10-/- mice, similar to Fbn1-/- mice, most likely because the mouse zonule contains fibrillin-2 in addition to fibrillin-1. Unexpectedly, in contrast to wild-type eyes, Adamts10-/- zonule fibers were thicker and immunostained strongly with fibrillin-2 antibodies into adulthood, whereas fibrillin-1 staining was reduced. Furthermore, fibrillin-2 staining of hyaloid vasculature remnants persisted post-natally in Adamts10-/- eyes. ADAMTS10 was found to cleave fibrillin-2, providing an explanation for persistence of fibrillin-2 at these sites. Thus, analysis of Adamts10-/- mice led to identification of fibrillin-2 as a novel ADAMTS10 substrate and defined a proteolytic mechanism for clearance of ocular fibrillin-2 at the end of the juvenile period.
Keywords: Acromelic dysplasia; Ectopia lentis; Extracellular matrix; Metalloprotease; Weill-Marchesani syndrome; Zonule.
Copyright © 2018 Elsevier B.V. All rights reserved.
Figures
References
-
- Davis EC, Roth RA, Heuser JE, Mecham RP, Ultrastructural properties of ciliary zonule microfibrils, J. Struct. Biol 139 (2) (2002) 65–75. - PubMed
-
- Reinhardt DP, Keene DR, Corson GM, Poschl E, Bachinger HP, Gambee JE, Sakai LY, Fibrillin-1: organization in microfibrils and structural properties, J. Mol. Biol 258 (1) (1996) 104–116. - PubMed
-
- Visconti RP, Barth JL, Keeley FW, Little CD, Codistribution analysis of elastin and related fibrillar proteins in early vertebrate development, Matrix Biol 22 (2) (2003) 109–121. - PubMed
-
- Corson GM, Charbonneau NL, Keene DR, Sakai LY, Differential expression of fibrillin-3 adds to microfibril variety in human and avian, but not rodent, connective tissues, Genomics 83 (3) (2004) 461–472. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

