A bone mineralization defect in the Pahenu2 model of classical phenylketonuria involves compromised mesenchymal stem cell differentiation
- PMID: 30201326
- PMCID: PMC6542264
- DOI: 10.1016/j.ymgme.2018.08.010
A bone mineralization defect in the Pahenu2 model of classical phenylketonuria involves compromised mesenchymal stem cell differentiation
Abstract
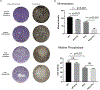
Osteopenia is observed in some patients affected by phenylalanine hydroxylase (PAH) deficient phenylketonuria (PKU). Bone density studies, in diverse PKU patient cohorts, have demonstrated bone disease is neither fully penetrant nor uniform in bone density loss. Biochemical assessment has generated a muddled perspective regarding mechanisms of the PKU bone phenotype where the participation of hyperphenylalaninemia remains unresolved. Osteopenia is realized in the Pahenu2 mouse model of classical PKU; although, characterization is incomplete. We characterized the Pahenu2 bone phenotype and assessed the effect of hyperphenylalaninemia on bone differentiation. Employing Pahenu2 and control animals, cytology, static and dynamic histomorphometry, and biochemistry were applied to further characterize the bone phenotype. These investigations demonstrate Pahenu2 bone density is decreased 33% relative to C57BL/6; bone volume/total volume was similarly decreased; trabecular thickness was unchanged while increased trabecular spacing was observed. Dynamic histomorphometry demonstrated a 25% decrease in mineral apposition. Biochemically, control and PKU animals have similar plasma cortisol, adrenocorticotropic hormone, and 25-hydroxyvitamin D. PKU animals show moderately increased plasma parathyroid hormone while plasma calcium and phosphate are reduced. These data are consistent with a mineralization defect. The effect of hyperphenylalaninemia on bone maturation was assessed in vitro employing bone-derived mesenchymal stem cells (MSCs) and their differentiation into bone. Using standard culture conditions, PAH deficient MSCs differentiate into bone as assessed by in situ alkaline phosphatase activity and mineral staining. However, PAH deficient MSCs cultured in 1200 μM PHE (metric defining classical PKU) show significantly reduced mineralization. These data are the first biological evidence demonstrating a negative impact of hyperphenylalaninemia upon bone maturation. In PAH deficient MSCs, expression of Col1A1 and Rankl are suppressed by hyperphenylalaninemia consistent with reduced bone formation and bone turnover. Osteopenia is intrinsic to PKU pathology in untreated Pahenu2 animals and our data suggests PHE toxicity participates by inhibiting mineralization in the course of MSC bone differentiation.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures


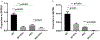
Similar articles
-
Phenylketonuria oxidative stress and energy dysregulation: Emerging pathophysiological elements provide interventional opportunity.Mol Genet Metab. 2022 Jun;136(2):111-117. doi: 10.1016/j.ymgme.2022.03.012. Epub 2022 Mar 29. Mol Genet Metab. 2022. PMID: 35379539 Free PMC article. Review.
-
Mesenchymal stem cell energy deficit and oxidative stress contribute to osteopenia in the Pahenu2 classical PKU mouse.Mol Genet Metab. 2021 Mar;132(3):173-179. doi: 10.1016/j.ymgme.2021.01.014. Epub 2021 Feb 11. Mol Genet Metab. 2021. PMID: 33602601 Free PMC article.
-
Glutamine energy substrate anaplerosis increases bone density in the Pahenu2 classical PKU mouse in the absence of phenylalanine restriction.JIMD Rep. 2022 Jul 6;63(5):446-452. doi: 10.1002/jmd2.12308. eCollection 2022 Sep. JIMD Rep. 2022. PMID: 36101821 Free PMC article.
-
DNA methylation in the pathophysiology of hyperphenylalaninemia in the PAH(enu2) mouse model of phenylketonuria.Mol Genet Metab. 2016 Sep;119(1-2):1-7. doi: 10.1016/j.ymgme.2016.01.001. Epub 2016 Jan 14. Mol Genet Metab. 2016. PMID: 26822703 Free PMC article.
-
Mutations in the phenylalanine hydroxylase gene: genetic determinants for the phenotypic variability of hyperphenylalaninemia.Acta Paediatr Suppl. 1994 Dec;407:49-56. doi: 10.1111/j.1651-2227.1994.tb13451.x. Acta Paediatr Suppl. 1994. PMID: 7766959 Review.
Cited by
-
Phenylketonuria oxidative stress and energy dysregulation: Emerging pathophysiological elements provide interventional opportunity.Mol Genet Metab. 2022 Jun;136(2):111-117. doi: 10.1016/j.ymgme.2022.03.012. Epub 2022 Mar 29. Mol Genet Metab. 2022. PMID: 35379539 Free PMC article. Review.
-
Plastrum testudinis Ameliorates Oxidative Stress in Nucleus Pulposus Cells via Downregulating the TNF-α Signaling Pathway.Pharmaceuticals (Basel). 2023 Oct 17;16(10):1482. doi: 10.3390/ph16101482. Pharmaceuticals (Basel). 2023. PMID: 37895953 Free PMC article.
-
Creatine energy substrate increases bone density in the Pahenu2 classical PKU mouse in the context of phenylalanine restriction.Mol Genet Metab Rep. 2023 Aug 6;36:100996. doi: 10.1016/j.ymgmr.2023.100996. eCollection 2023 Sep. Mol Genet Metab Rep. 2023. PMID: 37588420 Free PMC article.
-
Bone Status in Patients with Phenylketonuria: A Systematic Review.Nutrients. 2020 Jul 20;12(7):2154. doi: 10.3390/nu12072154. Nutrients. 2020. PMID: 32698408 Free PMC article.
-
The calcium channel Orai1 is required for osteoblast development: Studies in a chimeric mouse with variable in vivo Runx-cre deletion of Orai-1.PLoS One. 2023 May 11;18(5):e0264596. doi: 10.1371/journal.pone.0264596. eCollection 2023. PLoS One. 2023. PMID: 37167218 Free PMC article.
References
-
- Guthrie R, Sussi A, A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants, Pediatrics 32 (1963) 318–322. - PubMed
-
- Følling A, Uber Ausscheidung von Phenylbrenztraubensaure in den Harn als Stoffwechselanomalie in Verbindung mit Imbezillitat, Hoppe-Seylers Z Physiol Chem 277 (1934) 169.
-
- Jervis GA, Phenylpyruvic oligophrenia: Deficiency of phenylalanine oxidizing system, Proc. Soc. Exp. Biol. Med. 82 (1953) 514. - PubMed
-
- Bickel H, et al., Influence of phenylalanine intake on phenylketonuria, Lancet 265 (1953) 812. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

