Severity dependent distribution of impairments in PSP and CBS: Interactive visualizations
- PMID: 30201421
- PMCID: PMC6399076
- DOI: 10.1016/j.parkreldis.2018.08.025
Severity dependent distribution of impairments in PSP and CBS: Interactive visualizations
Abstract
Background: Progressive supranuclear palsy (PSP) -Richardson's Syndrome and Corticobasal Syndrome (CBS) are the two classic clinical syndromes associated with underlying four repeat (4R) tau pathology. The PSP Rating Scale is a commonly used assessment in PSP clinical trials; there is an increasing interest in designing combined 4R tauopathy clinical trials involving both CBS and PSP.
Objectives: To determine contributions of each domain of the PSP Rating Scale to overall severity and characterize the probable sequence of clinical progression of PSP as compared to CBS.
Methods: Multicenter clinical trial and natural history study data were analyzed from 545 patients with PSP and 49 with CBS. Proportional odds models were applied to model normalized cross-sectional PSP Rating Scale, estimating the probability that a patient would experience impairment in each domain using the PSP Rating Scale total score as the index of overall disease severity.
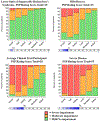
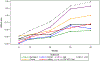
Results: The earliest symptom domain to demonstrate impairment in PSP patients was most likely to be Ocular Motor, followed jointly by Gait/Midline and Daily Activities, then Limb Motor and Mentation, and finally Bulbar. For CBS, Limb Motor manifested first and ocular showed less probability of impairment throughout the disease spectrum. An online tool to visualize predicted disease progression was developed to predict relative disability on each subscale per overall disease severity.
Conclusion: The PSP Rating Scale captures disease severity in both PSP and CBS. Modelling how domains change in relation to one other at varying disease severities may facilitate detection of therapeutic effects in future clinical trials.
Keywords: Corticobasal syndrome; Interactive visualizations; PSP rating scale; Predictive models; Progressive supranuclear palsy.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
DM receives income through UC San Francisco and has no other financial parties to disclose.
ALB receives research support from the NIH (R01AG038791, U54NS092089), the University of California, the Tau Consortium, CBD Solutions, the Bluefield Project to Cure FTD, the Alzheimer’s Association and the following companies: Avid, Biogen, Bristol Myers Squibb, C2N Diagnostics, Cortice Biosciences, Eli Lilly, Forum Pharmaceuticals, Genentech, Roche and TauRx; has served as a consultant for Abbvie, Asceneuron, Celgene, Ionis Pharmaceuticals, Janssen, Merck, Novartis, Samumed, Toyama and UCB; serves on a Data and Safety Monitoring Board for Neurogenetics Pharmaceuticals; has stock and/or options in Aeton, Alector and Delos.
TDS has not received research grants, has not served as a consultant and has not participated in any Advisory Board during the last year. He has no Intellectual Property Rights, Royalties, Contracts or Grants. He has stock in Pharmamar.
SL has received research grants from the Bavarian State and the Deutsche Stifterverband. He has received honoraria for presentations from UCB and Abbvie. He has not served as a consultant and has not participated in any Advisory Board during the last year. He has no Intellectual Property Rights, Royalties, Contracts stock ownerships.
GH has served on the advisory boards for AbbVie, Alzprotect, Asceneuron, Bristol-Myers Squibb, Novartis, Roche, Sellas Life Sciences Group, UCB; has received honoraria for scientific presentations from Abbvie, Roche, Teva, UCB, has received research support from CurePSP, the German Academic Exchange Service (DAAD), German Parkinson’s Disease Foundation (DPG), German PSP Association (PSP Gesellschaft), German Research Foundation (DFG) and the German Ministry of Education and Research (BMBF), International Parkinson’s Fonds (IPF), the Sellas Life Sciences Group; has received institutional support from the German Center for Neurodegenerative Diseases (DZNE).
GH was funded by the Deutsche Forschungsgemeinschaft (DFG, HO2402/6–2 & Munich Cluster for Systems Neurology SyNergy).
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

