Smartphone-Based Contingency Management Intervention to Improve Pre-Exposure Prophylaxis Adherence: Pilot Trial
- PMID: 30201601
- PMCID: PMC6231728
- DOI: 10.2196/10456
Smartphone-Based Contingency Management Intervention to Improve Pre-Exposure Prophylaxis Adherence: Pilot Trial
Abstract
Background: Pre-exposure prophylaxis (PrEP) provides a strong preventative benefit to individuals at risk for HIV. While PrEP adherence is highly correlated with its efficacy, adherence rates are variable both across and within persons.
Objective: The objective of this study was to develop and pilot-test a smartphone-based intervention, known as mSMART, that targets PrEP adherence. mSMART provides contingency management in the form of monetary incentives for daily PrEP adherence based on a real-time adherence assessment using a camera-based medication event-monitoring tool as well as medication reminders, PrEP education, individualized behavioral strategies to address PrEP adherence barriers, and medication adherence feedback.
Methods: This was a 4-week open-label, phase I trial in a community sample of young men who have sex with men already on PrEP (N=10).
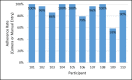
Results: Although adherence composite scores corresponding to PrEP biomarkers indicated that 90% (9/10) of the sample already had an acceptable baseline adherence in the protective range, by the end of the 4-week period, the scores improved for 30% (3/10) of the sample-adherence did not worsen for any participants. Participants reported mean PrEP adherence rates of 91% via daily entries in mSMART. At the end of the 4-week period, participants indicated acceptable ratings of satisfaction, usability, and willingness to recommend mSMART to others. There were no technical difficulties associated with smartphone compatibility, user misunderstandings about mSMART features that interfered with daily use, or study attrition.
Conclusions: This study is the first to apply contingency management to PrEP adherence. Findings indicated that mSMART is feasible and acceptable. Such an adherence intervention administered via a user-friendly smartphone app can allow for widespread dissemination. Future efficacy trials are needed.
Trial registration: ClinicalTrials.gov NCT02895893; https://clinicaltrials.gov/ct2/show/NCT02895893 (Accessed by Webcite at http://www.webcitation.org/72JskjDJq).
Keywords: HIV; mobile health; preexposure prophylaxis.
©John T Mitchell, Sara LeGrand, Lisa B Hightow-Weidman, Mehri S. McKellar, Angela DM Kashuba, Mackenzie Cottrell, Tony McLaurin, Goutam Satapathy, F Joseph McClernon. Originally published in JMIR Mhealth and Uhealth (http://mhealth.jmir.org), 10.09.2018.
Conflict of interest statement
Conflicts of Interest: JTM and FJM have served as site principal investigator and coinvestigator on a subcontract from Intelligent Automation Incorporated (IAI) funded by the National Institutes of Health (N44DA132236) to develop mSMART for a different indication (ie, smoking cessation). JTM also served as site principal investigator on a subcontract from Intelligent Automation Incorporated funded under N43DA130022. GS is employed by IAI and represents IAI as mSMART product owner.
Figures



References
-
- Grant R, Anderson P, McMahan V, Liu A, Amico K, Mehrotra M. Results of the iPrEx open-label extension (iPrEx OLE) in men and transgender women who have sex with men: PrEP uptake, sexual practices, and HIV incidence. International AIDS Conference; 2014; Melbourne, Australia.
-
- Molina J, Capitant C, Charreau I, Meyer L, Spire B, Pialoux G. On demand PrEP with oral TDF-FTC in MSM: results of the ANRS Ipergay Trial. Conference on Retroviruses Opportunistic Infections; 2015; Seattle, WA.
-
- Grant RM, Anderson PL, McMahan V, Liu A, Amico KR, Mehrotra M, Hosek S, Mosquera C, Casapia M, Montoya O, Buchbinder S, Veloso VG, Mayer K, Chariyalertsak S, Bekker L, Kallas EG, Schechter M, Guanira J, Bushman L, Burns DN, Rooney JF, Glidden DV, iPrEx ST. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014 Sep;14(9):820–9. doi: 10.1016/S1473-3099(14)70847-3.S1473-3099(14)70847-3 - DOI - PMC - PubMed
-
- Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, Goicochea P, Casapía M, Guanira-Carranza JV, Ramirez-Cardich ME, Montoya-Herrera O, Fernández T, Veloso VG, Buchbinder SP, Chariyalertsak S, Schechter M, Bekker L, Mayer KH, Kallás EG, Amico KR, Mulligan K, Bushman LR, Hance RJ, Ganoza C, Defechereux P, Postle B, Wang F, McConnell JJ, Zheng J, Lee J, Rooney JF, Jaffe HS, Martinez AI, Burns DN, Glidden DV, iPrEx ST. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010 Dec 30;363(27):2587–99. doi: 10.1056/NEJMoa1011205. http://europepmc.org/abstract/MED/21091279 - DOI - PMC - PubMed
-
- Baeten J, Heffron R, Kidoguchi L, Celum C. Near elimination of HIV transmission in a demonstration project of PrEP and ART. Conference on Retroviruses Opportunistic Infections; 2015; Seattle, WA.
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

