Superoxide dismutase activity confers (p)ppGpp-mediated antibiotic tolerance to stationary-phase Pseudomonas aeruginosa
- PMID: 30201715
- PMCID: PMC6166797
- DOI: 10.1073/pnas.1804525115
Superoxide dismutase activity confers (p)ppGpp-mediated antibiotic tolerance to stationary-phase Pseudomonas aeruginosa
Abstract
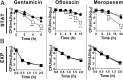
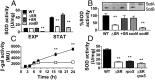
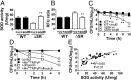
Metabolically quiescent bacteria represent a large proportion of those in natural and host environments, and they are often refractory to antibiotic treatment. Such drug tolerance is also observed in the laboratory during stationary phase, when bacteria face stress and starvation-induced growth arrest. Tolerance requires (p)ppGpp signaling, which mediates the stress and starvation stringent response (SR), but the downstream effectors that confer tolerance are unclear. We previously demonstrated that the SR is linked to increased antioxidant defenses in Pseudomonas aeruginosa We now demonstrate that superoxide dismutase (SOD) activity is a key factor in SR-mediated multidrug tolerance in stationary-phase P. aeruginosa Inactivation of the SR leads to loss of SOD activity and decreased multidrug tolerance during stationary phase. Genetic or chemical complementation of SOD activity of the ΔrelA spoT mutant (ΔSR) is sufficient to restore antibiotic tolerance to WT levels. Remarkably, we observe high membrane permeability and increased drug internalization upon ablation of SOD activity. Combined, our results highlight an unprecedented mode of SR-mediated multidrug tolerance in stationary-phase P. aeruginosa and suggest that inhibition of SOD activity may potentiate current antibiotics.
Keywords: (p)ppGpp stringent response; Pseudomonas aeruginosa; antibiotic tolerance; stationary phase; superoxide dismutase.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
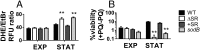

References
-
- Levin BR, Rozen DE. Non-inherited antibiotic resistance. Nat Rev Microbiol. 2006;4:556–562. - PubMed
-
- Meylan S, Andrews IW, Collins JJ. Targeting antibiotic tolerance, pathogen bypathogen. Cell. 2018;172:1228–1238. - PubMed
-
- Levin-Reisman I, et al. Antibiotic tolerance facilitates the evolution of resistance. Science. 2017;355:826–830. - PubMed
-
- Brauner A, Fridman O, Gefen O, Balaban NQ. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat Rev Microbiol. 2016;14:320–330. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

