Efficacy of PD-1 or PD-L1 inhibitors and PD-L1 expression status in cancer: meta-analysis
- PMID: 30201790
- PMCID: PMC6129950
- DOI: 10.1136/bmj.k3529
Efficacy of PD-1 or PD-L1 inhibitors and PD-L1 expression status in cancer: meta-analysis
Abstract
Objective: To evaluate the relative efficacy of programmed cell death 1 (PD-1) or programmed cell death ligand 1 (PD-L1) inhibitors versus conventional drugs in patients with cancer that were PD-L1 positive and PD-L1 negative.
Design: Meta-analysis of randomised controlled trials.
Data sources: PubMed, Embase, Cochrane database, and conference abstracts presented at the American Society of Clinical Oncology and European Society of Medical Oncology up to March 2018.
Review methods: Studies of PD-1 or PD-L1 inhibitors (avelumab, atezolizumab, durvalumab, nivolumab, and pembrolizumab) that had available hazard ratios for death based on PD-L1 positivity or negativity were included. The threshold for PD-L1 positivity or negativity was that PD-L1 stained cell accounted for 1% of tumour cells, or tumour and immune cells, assayed by immunohistochemistry staining methods.
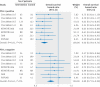
Results: 4174 patients with advanced or metastatic cancers from eight randomised controlled trials were included in this study. Compared with conventional agents, PD-1 or PD-L1 inhibitors were associated with significantly prolonged overall survival in both patients that were PD-L1 positive (n=2254, hazard ratio 0.66, 95% confidence interval 0.59 to 0.74) and PD-L1 negative (1920, 0.80, 0.71 to 0.90). However, the efficacies of PD-1 or PD-L1 blockade treatment in patients that were PD-L1 positive and PD-L1 negative were significantly different (P=0.02 for interaction). Additionally, in both patients that were PD-L1 positive and PD-L1 negative, the long term clinical benefits from PD-1 or PD-L1 blockade were observed consistently across interventional agent, cancer histotype, method of randomisation stratification, type of immunohistochemical scoring system, drug target, type of control group, and median follow-up time.
Conclusions: PD-1 or PD-L1 blockade therapy is a preferable treatment option over conventional therapy for both patients that are PD-L1 positive and PD-L1 negative. This finding suggests that PD-L1 expression status alone is insufficient in determining which patients should be offered PD-1 or PD-L1 blockade therapy.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE form disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
