Population Pharmacokinetics of Tafenoquine, a Novel Antimalarial
- PMID: 30201820
- PMCID: PMC6201082
- DOI: 10.1128/AAC.00711-18
Population Pharmacokinetics of Tafenoquine, a Novel Antimalarial
Abstract
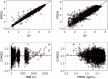
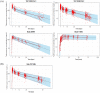
Tafenoquine is a novel 8-aminoquinoline antimalarial drug recently approved by the U.S. Food and Drug Administration (FDA) for the radical cure of acute Plasmodium vivax malaria, which is the first new treatment in almost 60 years. A population pharmacokinetic (POP PK) analysis was conducted with tafenoquine exposure data obtained following oral administration from 6 clinical studies in phase 1 through phase 3 with a nonlinear mixed effects modeling approach. The impacts of patient demographics, baseline characteristics, and extrinsic factors, such as formulation, were evaluated. Model performance was assessed using techniques such as bootstrapping, visual predictive checks, and external data validation from a phase 3 study not used in model fitting and parameter estimation. Based on the analysis, the systemic pharmacokinetics of tafenoquine were adequately described using a two-compartment model. The final POP PK model included body weight (allometric scaling) on apparent oral and intercompartmental clearance (CL/F and Q/F, respectively), apparent volume of distribution for central and peripheral compartments (V2/F and V3/F, respectively), formulation on systemic bioavailability (F1) and absorption rate constant (Ka ), and health status on apparent volume of distribution. The key tafenoquine population parameter estimates were 2.96 liters/h for CL/F and 915 liters for V2/F in P. vivax-infected subjects. Additionally, the analyses demonstrated no clinically relevant difference in relative bioavailability across the capsule and tablet formulations administered in these clinical studies. In conclusion, a POP PK model for tafenoquine was developed. Clinical trial simulations based on this model supported bridging the exposures across two different formulations. This POP PK model can be applied to aid and perform clinical trial simulations in other scenarios and populations, such as pediatric populations.
Keywords: 8-aminoquinoline; antimalarial; population pharmacokinetics; tafenoquine.
Copyright © 2018 Thakkar et al.
Figures

References
-
- World Health Organization. 2016. World malaria report 2016. World Health Organization, Geneva, Switzerland.
-
- World Health Organization. Guidelines for the treatment of malaria. 2015. World Health Organization, Geneva, Switzerland.
-
- Abreha T, Hwang J, Thriemer K, Tadesse Y, Girma S, Melaku Z, Assef A, Kassa M, Chatfield MD, Landman KZ, Chenet SM, Lucchi NW, Udhayakumar V, Zhou Z, Shi YP, Kachur SP, Jima D, Kebede A, Solomon H, Mekasha A, Alemayehu BH, Malone JL, Dissanayake G, Teka H, Auburn S, von Seidlein L, Price RN. 2017. Comparison of artemether-lumefantrine and chloroquine with and without primaquine for the treatment of Plasmodium vivax infection in Ethiopia: a randomized controlled trial. PLoS Med 14:e1002299. doi: 10.1371/journal.pmed.1002299. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

