CIK Receptor Kinases Determine Cell Fate Specification during Early Anther Development in Arabidopsis
- PMID: 30201822
- PMCID: PMC6241272
- DOI: 10.1105/tpc.17.00586
CIK Receptor Kinases Determine Cell Fate Specification during Early Anther Development in Arabidopsis
Abstract
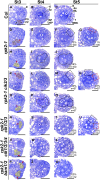
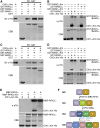
Appropriate cell division and differentiation ensure normal anther development in angiosperms. BARELY ANY MERISTEM 1/2 (BAM1/2) and RECEPTOR-LIKE PROTEIN KINASE2 (RPK2), two groups of leucine-rich repeat receptor-like protein kinases, are required for early anther cell specification. However, little is known about the molecular mechanisms underlying these two RLK-mediated signaling pathways. Here, we show that CLAVATA3 INSENSITIVE RECEPTOR KINASEs (CIKs), a group of novel coreceptor protein kinase-controlling stem cell homeostasis, play essential roles in BAM1/2- and RPK2-regulated early anther development in Arabidopsis thaliana The archesporial cells of cik1/2/3 triple and cik1/2/3/4 quadruple mutant anthers perform anticlinal division instead of periclinal division. Defective cell division and specification of the primary and inner secondary parietal cells occur in these mutant anthers. The disordered divisions and specifications of anther wall cells finally result in excess microsporocytes and a lack of one to three parietal cell layers in mutant anthers, resembling rpk2 or bam1/2 mutant anthers. Genetic and biochemical analyses indicate that CIKs function as coreceptors of BAM1/2 and RPK2 to regulate archesporial cell division and determine the specification of anther parietal cells.
© 2018 American Society of Plant Biologists. All rights reserved.
Figures




Similar articles
-
The BAM1/BAM2 receptor-like kinases are important regulators of Arabidopsis early anther development.Plant Cell. 2006 Jul;18(7):1667-80. doi: 10.1105/tpc.105.036871. Epub 2006 Jun 2. Plant Cell. 2006. PMID: 16751349 Free PMC article.
-
Receptor-like protein kinase 2 (RPK 2) is a novel factor controlling anther development in Arabidopsis thaliana.Plant J. 2007 Jun;50(5):751-66. doi: 10.1111/j.1365-313X.2007.03083.x. Epub 2007 Apr 5. Plant J. 2007. PMID: 17419837
-
Regulation of Arabidopsis early anther development by the mitogen-activated protein kinases, MPK3 and MPK6, and the ERECTA and related receptor-like kinases.Mol Plant. 2008 Jul;1(4):645-58. doi: 10.1093/mp/ssn029. Epub 2008 Jun 3. Mol Plant. 2008. PMID: 19825569
-
Control of anther cell differentiation: a teamwork of receptor-like kinases.Sex Plant Reprod. 2009 Dec;22(4):221-8. doi: 10.1007/s00497-009-0106-3. Epub 2009 Aug 6. Sex Plant Reprod. 2009. PMID: 20033443 Review.
-
Pre-Meiotic Anther Development: Cell Fate Specification and Differentiation.Annu Rev Plant Biol. 2016 Apr 29;67:365-95. doi: 10.1146/annurev-arplant-043015-111804. Epub 2016 Jan 6. Annu Rev Plant Biol. 2016. PMID: 26735065 Review.
Cited by
-
WOX going on: CLE peptides in plant development.Curr Opin Plant Biol. 2021 Oct;63:102056. doi: 10.1016/j.pbi.2021.102056. Epub 2021 May 30. Curr Opin Plant Biol. 2021. PMID: 34077886 Free PMC article. Review.
-
Anther development in Arabidopsis thaliana involves symplastic isolation and apoplastic gating of the tapetum-middle layer interface.Development. 2022 Nov 15;149(22):dev200596. doi: 10.1242/dev.200596. Epub 2022 Nov 16. Development. 2022. PMID: 36305487 Free PMC article.
-
Receptor-like cytoplasmic kinases PBL34/35/36 are required for CLE peptide-mediated signaling to maintain shoot apical meristem and root apical meristem homeostasis in Arabidopsis.Plant Cell. 2022 Mar 29;34(4):1289-1307. doi: 10.1093/plcell/koab315. Plant Cell. 2022. PMID: 34935965 Free PMC article.
-
Male Germ Cell Specification in Plants.Int J Mol Sci. 2024 Jun 17;25(12):6643. doi: 10.3390/ijms25126643. Int J Mol Sci. 2024. PMID: 38928348 Free PMC article. Review.
-
Global transcriptome analysis reveals potential genes associated with genic male sterility of rapeseed (Brassica napus L.).Front Plant Sci. 2022 Oct 21;13:1004781. doi: 10.3389/fpls.2022.1004781. eCollection 2022. Front Plant Sci. 2022. PMID: 36340380 Free PMC article.
References
-
- Alexander M.P. (1969). Differential staining of aborted and nonaborted pollen. Stain Technol. 44: 117–122. - PubMed
-
- Brand U., Fletcher J.C., Hobe M., Meyerowitz E.M., Simon R. (2000). Dependence of stem cell fate in Arabidopsis on a feedback loop regulated by CLV3 activity. Science 289: 617–619. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

