Caenorhabditis elegans and the network control framework-FAQs
- PMID: 30201837
- PMCID: PMC6158218
- DOI: 10.1098/rstb.2017.0372
Caenorhabditis elegans and the network control framework-FAQs
Abstract
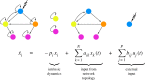
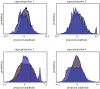
Control is essential to the functioning of any neural system. Indeed, under healthy conditions the brain must be able to continuously maintain a tight functional control between the system's inputs and outputs. One may therefore hypothesize that the brain's wiring is predetermined by the need to maintain control across multiple scales, maintaining the stability of key internal variables, and producing behaviour in response to environmental cues. Recent advances in network control have offered a powerful mathematical framework to explore the structure-function relationship in complex biological, social and technological networks, and are beginning to yield important and precise insights on neuronal systems. The network control paradigm promises a predictive, quantitative framework to unite the distinct datasets necessary to fully describe a nervous system, and provide mechanistic explanations for the observed structure and function relationships. Here, we provide a thorough review of the network control framework as applied to Caenorhabditis elegans (Yan et al. 2017 Nature550, 519-523. (doi:10.1038/nature24056)), in the style of Frequently Asked Questions. We present the theoretical, computational and experimental aspects of network control, and discuss its current capabilities and limitations, together with the next likely advances and improvements. We further present the Python code to enable exploration of control principles in a manner specific to this prototypical organism.This article is part of a discussion meeting issue 'Connectome to behaviour: modelling C. elegans at cellular resolution'.
Keywords: C. elegans; connectome; control theory; locomotion; network science.
© 2018 The Author(s).
Conflict of interest statement
We have no competing interests.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
