From head to tail: a neuromechanical model of forward locomotion in Caenorhabditis elegans
- PMID: 30201838
- PMCID: PMC6158225
- DOI: 10.1098/rstb.2017.0374
From head to tail: a neuromechanical model of forward locomotion in Caenorhabditis elegans
Abstract
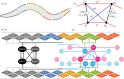
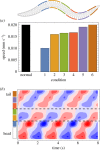
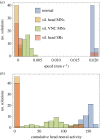
With 302 neurons and a near-complete reconstruction of the neural and muscle anatomy at the cellular level, Caenorhabditis elegans is an ideal candidate organism to study the neuromechanical basis of behaviour. Yet despite the breadth of knowledge about the neurobiology, anatomy and physics of C. elegans, there are still a number of unanswered questions about one of its most basic and fundamental behaviours: forward locomotion. How the rhythmic pattern is generated and propagated along the body is not yet well understood. We report on the development and analysis of a model of forward locomotion that integrates the neuroanatomy, neurophysiology and body mechanics of the worm. Our model is motivated by experimental analysis of the structure of the ventral cord circuitry and the effect of local body curvature on nearby motoneurons. We developed a neuroanatomically grounded model of the head motoneuron circuit and the ventral nerve cord circuit. We integrated the neural model with an existing biomechanical model of the worm's body, with updated musculature and stretch receptors. Unknown parameters were evolved using an evolutionary algorithm to match the speed of the worm on agar. We performed 100 evolutionary runs and consistently found electrophysiological configurations that reproduced realistic control of forward movement. The ensemble of successful solutions reproduced key experimental observations that they were not designed to fit, including the wavelength and frequency of the propagating wave. Analysis of the ensemble revealed that head motoneurons SMD and RMD are sufficient to drive dorsoventral undulations in the head and neck and that short-range posteriorly directed proprioceptive feedback is sufficient to propagate the wave along the rest of the body.This article is part of a discussion meeting issue 'Connectome to behaviour: modelling C. elegans at cellular resolution'.
Keywords: invertebrate; locomotion; motor control; neuromechanical model; proprioception.
© 2018 The Author(s).
Conflict of interest statement
We declare we have no competing interests.
Figures



References
-
- Waterston RH. 1988. Muscle In The nematode C. elegans (ed. Wood WB.), pp. 281–335. New York, NY: Cold Spring Harbor Laboratory Press.
-
- Babington P. 1997. C. elegans II, 2nd edn New York, NY: Cold Spring Harbour Laboratory Press.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
