Signatures of proprioceptive control in Caenorhabditis elegans locomotion
- PMID: 30201846
- PMCID: PMC6158217
- DOI: 10.1098/rstb.2018.0208
Signatures of proprioceptive control in Caenorhabditis elegans locomotion
Abstract
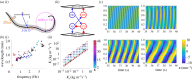
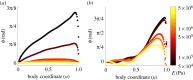
Animal neuromechanics describes the coordinated self-propelled movement of a body, subject to the combined effects of internal neural control and mechanical forces. Here we use a computational model to identify effects of neural and mechanical modulation on undulatory forward locomotion of Caenorhabditis elegans, with a focus on proprioceptively driven neural control. We reveal a fundamental relationship between body elasticity and environmental drag in determining the dynamics of the body and demonstrate the manifestation of this relationship in the context of proprioceptively driven control. By considering characteristics unique to proprioceptive neurons, we predict the signatures of internal gait modulation that contrast with the known signatures of externally or biomechanically modulated gait. We further show that proprioceptive feedback can suppress neuromechanical phase lags during undulatory locomotion, contrasting with well studied advancing phase lags that have long been a signature of centrally generated, feed-forward control.This article is part of a discussion meeting issue 'Connectome to behaviour: modelling C. elegans at cellular resolution'.
Keywords: microswimmers; nematodes; neural control; proprioception; undulatory locomotion.
© 2018 The Author(s).
Conflict of interest statement
We declare we have no competing interests.
Figures



References
-
- Lighthill J. 1976. Flagellar hydrodynamics. SIAM Rev. 18, 161–230. ( 10.1137/1018040) - DOI
-
- Gray J, Lissmann HW. 1964. The locomotion of nematodes. J. Exp. Biol. 41, 135–154. - PubMed
-
- Wallace H. 1969. Wave formation by infective larvae of the plant parasitic nematode Meloidogyne javanica. Nematologica 15, 65–75. ( 10.1163/187529269X00100) - DOI
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
