The Role of Sugarcane Catalase Gene ScCAT2 in the Defense Response to Pathogen Challenge and Adversity Stress
- PMID: 30201878
- PMCID: PMC6163996
- DOI: 10.3390/ijms19092686
The Role of Sugarcane Catalase Gene ScCAT2 in the Defense Response to Pathogen Challenge and Adversity Stress
Abstract
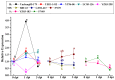
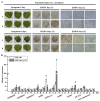
Catalases, which consist of multiple structural isoforms, catalyze the decomposition of hydrogen peroxide in cells to prevent membrane lipid peroxidation. In this study, a group II catalase gene ScCAT2 (GenBank Accession No. KF528830) was isolated from sugarcane genotype Yacheng05-179. ScCAT2 encoded a predicted protein of 493 amino acid residues, including a catalase active site signature (FARERIPERVVHARGAS) and a heme-ligand signature (RVFAYADTQ). Subcellular localization experiments showed that the ScCAT2 protein was distributed in the cytoplasm, plasma membrane, and nucleus of Nicotiana benthamiana epidermal cells. Quantitative real-time polymerase chain reaction (qRT-PCR) analysis indicated that the ScCAT2 gene was ubiquitously expressed in sugarcane tissues, with expression levels from high to low in stem skin, stem pith, roots, buds, and leaves. ScCAT2 mRNA expression was upregulated after treatment with abscisic acid (ABA), sodium chloride (NaCl), polyethylene glycol (PEG), and 4 °C low temperature, but downregulated by salicylic acid (SA), methyl jasmonate (MeJA), and copper chloride (CuCl₂). Moreover, tolerance of Escherichia coli Rosetta cells carrying pET-32a-ScCAT2 was enhanced by NaCl stress, but not by CuCl₂ stress. Sporisorium scitamineum infection of 10 different sugarcane genotypes showed that except for YZ03-258, FN40, and FN39, ScCAT2 transcript abundance in four smut-resistant cultivars (Yacheng05-179, YZ01-1413, YT96-86, and LC05-136) significantly increased at the early stage (1 day post-inoculation), and was decreased or did not change in the two smut-medium-susceptibility cultivars (ROC22 and GT02-467), and one smut-susceptible cultivar (YZ03-103) from 0 to 3 dpi. Meanwhile, the N. benthamiana leaves that transiently overexpressed ScCAT2 exhibited less severe disease symptoms, more intense 3,3'-diaminobenzidine (DAB) staining, and higher expression levels of tobacco immune-related marker genes than the control after inoculation with tobacco pathogen Ralstonia solanacearum or Fusarium solani var. coeruleum. These results indicate that ScCAT2 plays a positive role in immune responses during plant⁻pathogen interactions, as well as in salt, drought, and cold stresses.
Keywords: Sporisorium scitamineum; catalase; defense response; expression profiles; sugarcane.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Willekens H., Inzé D., Montagu M.V., Camp W.V. Catalases in plants. Mol. Breed. 1995;1:207–228. doi: 10.1007/BF02277422. - DOI
-
- Iwamoto M., Higo H., Higo K. Differential diurnal expression of rice catalase genes: The 5′-flanking region of CatA, is not sufficient for circadian control. Plant Sci. 2000;151:39–46. doi: 10.1016/S0168-9452(99)00194-6. - DOI
MeSH terms
Substances
Grants and funding
- 31501363, 31101196 and 31671752/National Natural Science Foundation of China
- SYC-2017/the Research Funds for Distinguished Young Scientists in Fujian Provincial Department of Education
- KFA17267A/the Special Fund for Science and Technology Innovation of Fujian Agriculture and Forestry University
- CARS-17/the China Agriculture Research System
LinkOut - more resources
Full Text Sources
Other Literature Sources

