Formulation and Evaluation of Silymarin-Loaded Chitosan-Montmorilloite Microbeads for the Potential Treatment of Gastric Ulcers
- PMID: 30201932
- PMCID: PMC6164251
- DOI: 10.3390/jfb9030052
Formulation and Evaluation of Silymarin-Loaded Chitosan-Montmorilloite Microbeads for the Potential Treatment of Gastric Ulcers
Abstract
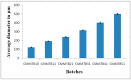
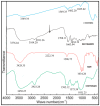
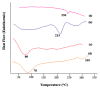
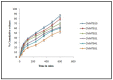
Silymarin-loaded mucoadhesive microbeads of Chitosan-MMT were developed using the ionotropic gelation technique. Characterization of the microbeads was performed by DSC, XRD, SEM, and FTIR techniques. In vitro mucoadhesion and drug release studies; gastroprotective studies including the measurement of ulcerative index; the determination of gastric wall mucus; and the determination of percentage protection, biochemical, and histopathological studies were also performed. Microbeads batches were evaluated for particle size (120⁻140 µm), actual drug content, (49.36⁻58.18%) and entrapment efficiency (72.52⁻92.39%).Biochemical estimation of myeloperoxidase was found to be 0.10⁻0.75 µmoles/g/tissue. Significant reduction in the ulcerative index showed the gastroprotective effect of the formulation. Silymarin-loaded beads of Chitosan-MMT were found to exhibit good mucoadhesion and efficient release of the drug, and were found to be a promising drug carrier system for the treatment of gastric ulcers.
Keywords: Microbeads; chitosan; gastric ulcers; montmorillonite; silymarin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Fabrication and in vitro evaluation of mucoadhesive ondansetron hydrochloride beads for the management of emesis in chemotherapy.Int J Pharm Investig. 2013 Jan;3(1):42-6. doi: 10.4103/2230-973X.108962. Int J Pharm Investig. 2013. PMID: 23799204 Free PMC article.
-
Floating-bioadhesive gastroretentive Caesalpinia pulcherrima-based beads of amoxicillin trihydrate for Helicobacter pylori eradication.Drug Deliv. 2016;23(2):405-19. doi: 10.3109/10717544.2014.916766. Epub 2014 May 28. Drug Deliv. 2016. PMID: 24870198
-
Chitosan-based mucoadhesive microspheres of clarithromycin as a delivery system for antibiotic to stomach.Curr Drug Deliv. 2005 Jul;2(3):235-42. doi: 10.2174/1567201054367995. Curr Drug Deliv. 2005. PMID: 16305425
-
Development of mucoadhesive microbeads using thiolated sodium alginate for intrapocket delivery of resveratrol.Int J Pharm. 2015 Jun 20;487(1-2):305-13. doi: 10.1016/j.ijpharm.2015.04.010. Epub 2015 Apr 9. Int J Pharm. 2015. PMID: 25865569
-
Enhancing Stability and Mucoadhesive Properties of Chitosan Nanoparticles by Surface Modification with Sodium Alginate and Polyethylene Glycol for Potential Oral Mucosa Vaccine Delivery.Mar Drugs. 2022 Feb 22;20(3):156. doi: 10.3390/md20030156. Mar Drugs. 2022. PMID: 35323455 Free PMC article.
Cited by
-
A Comprehensive Review on Nutraceuticals: Therapy Support and Formulation Challenges.Nutrients. 2022 Nov 3;14(21):4637. doi: 10.3390/nu14214637. Nutrients. 2022. PMID: 36364899 Free PMC article. Review.
-
The Effect of Halloysite Addition on the Material Properties of Chitosan-Halloysite Hydrogel Composites.Gels. 2019 Aug 14;5(3):40. doi: 10.3390/gels5030040. Gels. 2019. PMID: 31416252 Free PMC article.
-
Formulation of Folate Receptor-Targeted Silibinin-Loaded Inhalable Chitosan Nanoparticles by the QbD Approach for Lung Cancer Targeted Delivery.ACS Omega. 2024 Feb 24;9(9):10353-10370. doi: 10.1021/acsomega.3c07954. eCollection 2024 Mar 5. ACS Omega. 2024. PMID: 38463259 Free PMC article.
-
Phytoconstituent-Loaded Nanofibrous Meshes as Wound Dressings: A Concise Review.Pharmaceutics. 2023 Mar 24;15(4):1058. doi: 10.3390/pharmaceutics15041058. Pharmaceutics. 2023. PMID: 37111544 Free PMC article. Review.
-
Preparation and Characterization of Silymarin Gel: A Novel Topical Mucoadhesive Formulation for Potential Applicability in Oral Pathologies.Gels. 2023 Feb 7;9(2):139. doi: 10.3390/gels9020139. Gels. 2023. PMID: 36826309 Free PMC article.
References
-
- Salcedo I., Aguzzi C., Sandri S. In vitro biocompatibility and mucoadhesion of montmorillonite chitosan nanocomposite: A new drug delivery. Appl. Clay Sci. 2012;55:131–137. doi: 10.1016/j.clay.2011.11.006. - DOI
-
- Xu Y., Ren X., Hanna M.A. Chitosan/clay nanocomposite film preparation and characterization. J. Appl. Polym. Sci. 2006;99:1684–1691. doi: 10.1002/app.22664. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

