Co-overexpression of native phospholipid-biosynthetic genes plsX and plsC enhances lipid production in Synechocystis sp. PCC 6803
- PMID: 30201972
- PMCID: PMC6131169
- DOI: 10.1038/s41598-018-31789-5
Co-overexpression of native phospholipid-biosynthetic genes plsX and plsC enhances lipid production in Synechocystis sp. PCC 6803
Abstract
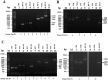
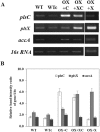
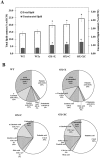
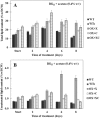
The overexpression of native plsX and plsC genes involving in fatty acid/phospholipid synthesis first timely-reported the significantly enhanced lipid contents in Synechocystis sp. PCC 6803. Growth rate, intracellular pigment contents including chlorophyll a and carotenoids, and oxygen evolution rate of all overexpressing (OX) strains were normally similar as wild type. For fatty acid compositions, saturated fatty acid, in particular palmitic acid (16:0) was dominantly increased in OX strains whereas slight increases of unsaturated fatty acids were observed, specifically linoleic acid (18:2) and alpha-linolenic acid (18:3). The plsC/plsX-overexpressing (OX + XC) strain produced high lipid content of about 24.3%w/dcw under normal condition and was further enhanced up to 39.1%w/dcw by acetate induction. This OX + XC engineered strain was capable of decreasing phaA transcript level which related to poly-3-hydroxybutyrate (PHB) synthesis under acetate treatment. Moreover, the expression level of gene transcripts revealed that the plsX- and plsC/plsX-overexpression strains had also increased accA transcript amounts which involved in the irreversible carboxylation of acetyl-CoA to malonyl-CoA. Altogether, these overexpressing strains significantly augmented higher lipid contents when compared to wild type by partly overcoming the limitation of lipid production.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Enhancement of poly-3-hydroxybutyrate production in Synechocystis sp. PCC 6803 by overexpression of its native biosynthetic genes.Bioresour Technol. 2016 Aug;214:761-768. doi: 10.1016/j.biortech.2016.05.014. Epub 2016 May 7. Bioresour Technol. 2016. PMID: 27213577
-
Synechocystis sp. PCC 6803 overexpressing genes involved in CBB cycle and free fatty acid cycling enhances the significant levels of intracellular lipids and secreted free fatty acids.Sci Rep. 2020 Mar 11;10(1):4515. doi: 10.1038/s41598-020-61100-4. Sci Rep. 2020. PMID: 32161307 Free PMC article.
-
Overexpression of fatty acid synthesis genes in Synechocystis sp. PCC 6803 with disrupted glycogen synthesis increases lipid production with further enhancement under copper induced oxidative stress.Chemosphere. 2022 Mar;291(Pt 1):132755. doi: 10.1016/j.chemosphere.2021.132755. Epub 2021 Nov 1. Chemosphere. 2022. PMID: 34736940
-
Thematic review series: Glycerolipids. Acyltransferases in bacterial glycerophospholipid synthesis.J Lipid Res. 2008 Sep;49(9):1867-74. doi: 10.1194/jlr.R800005-JLR200. Epub 2008 Mar 27. J Lipid Res. 2008. PMID: 18369234 Free PMC article. Review.
-
Phosphatidic acid synthesis in bacteria.Biochim Biophys Acta. 2013 Mar;1831(3):495-502. doi: 10.1016/j.bbalip.2012.08.018. Epub 2012 Aug 30. Biochim Biophys Acta. 2013. PMID: 22981714 Free PMC article. Review.
Cited by
-
Incorporation, fate, and turnover of free fatty acids in cyanobacteria.FEMS Microbiol Rev. 2023 Mar 10;47(2):fuad015. doi: 10.1093/femsre/fuad015. FEMS Microbiol Rev. 2023. PMID: 37061785 Free PMC article. Review.
-
Highlighting the potential of Synechococcus elongatus PCC 7942 as platform to produce α-linolenic acid through an updated genome-scale metabolic modeling.Front Microbiol. 2023 Mar 14;14:1126030. doi: 10.3389/fmicb.2023.1126030. eCollection 2023. Front Microbiol. 2023. PMID: 36998399 Free PMC article.
-
Induced Mutagenesis and Comparative Genomics of Raoultella sp. 64 for Enhanced Antimony Resistance and Biosorption.Microorganisms. 2025 Apr 11;13(4):880. doi: 10.3390/microorganisms13040880. Microorganisms. 2025. PMID: 40284716 Free PMC article.
-
Improved lipid production via fatty acid biosynthesis and free fatty acid recycling in engineered Synechocystis sp. PCC 6803.Biotechnol Biofuels. 2019 Jan 4;12:8. doi: 10.1186/s13068-018-1349-8. eCollection 2019. Biotechnol Biofuels. 2019. PMID: 30622650 Free PMC article.
-
Overexpression of lipA or glpD_RuBisCO in the Synechocystis sp. PCC 6803 Mutant Lacking the Aas Gene Enhances Free Fatty-Acid Secretion and Intracellular Lipid Accumulation.Int J Mol Sci. 2021 Oct 25;22(21):11468. doi: 10.3390/ijms222111468. Int J Mol Sci. 2021. PMID: 34768898 Free PMC article.
References
-
- Heyer H, Krumbein WE. Excretion of Fermentation Products in Dark and Anaerobically Incubated Cyanobacteria. Archives of Microbiology. 1991;155:284–287. doi: 10.1007/BF00252213. - DOI
-
- Karatay SE, Dömmez G. Microbial Oil Production from Thermophile Cyanobacteria for Biodiesel Production. Applied Energy. 2011;88:3632–3635. doi: 10.1016/j.apenergy.2011.04.010. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

